Determining clinically meaningful decline in preclinical Alzheimer disease
- PMID: 31289148
- PMCID: PMC6669933
- DOI: 10.1212/WNL.0000000000007831
Determining clinically meaningful decline in preclinical Alzheimer disease
Abstract
Objective: To determine the time required for a preclinical Alzheimer disease population to decline in a meaningful way, use estimates of decline to update previous clinical trial design assumptions, and identify factors that modify β-amyloid (Aβ)-related decline.
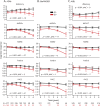
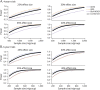
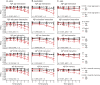
Methods: In 1,120 cognitively unimpaired individuals from 3 international cohorts, we estimated the relationship between Aβ status and longitudinal changes across multiple cognitive domains and assessed interactions between Aβ and baseline factors. Power analyses were performed to explore sample size as a function of treatment effect.
Results: Cognitively unimpaired Aβ+ participants approach mild cognitive impairment (MCI) levels of performance 6 years after baseline, on average. Achieving 80% power in a simulated 4-year treatment trial, assuming a 25% treatment effect, required 2,000 participants/group. Multiple factors interacted with Aβ to predict cognitive decline; however, these findings were all cohort-specific. Despite design differences across the cohorts, with large sample sizes and sufficient follow-up time, the Aβ+ groups declined consistently on cognitive composite measures.
Conclusions: A preclinical AD population declines to the cognitive performance of an early MCI population in 6 years. Slowing this rate of decline by 40%-50% delays clinically relevant impairment by 3 years-a potentially meaningful treatment effect. However, assuming a 40%-50% drug effect highlights the difficulties in preclinical AD trial design, as a more commonly assumed treatment effect of 25% results in a required sample size of 2,000/group. Designers of preclinical AD treatment trials need to prepare for larger and longer trials than are currently being considered. Interactions with Aβ status were inconsistent and not readily generalizable.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

Comment in
-
The search for meaning in preclinical Alzheimer disease clinical trials.Neurology. 2019 Jul 23;93(4):139-140. doi: 10.1212/WNL.0000000000007817. Epub 2019 Jul 9. Neurology. 2019. PMID: 31289145 No abstract available.
