Sirtuin Inhibitors Are Broadly Antiviral against Arboviruses
- PMID: 31289184
- PMCID: PMC6747726
- DOI: 10.1128/mBio.01446-19
Sirtuin Inhibitors Are Broadly Antiviral against Arboviruses
Abstract
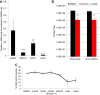
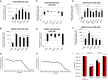
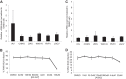
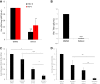
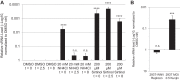
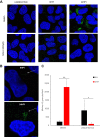
Arthropod-borne viruses are diverse pathogens and are often associated with human disease. These viruses span multiple genera, including flaviviruses, alphaviruses, and bunyaviruses. In a high-throughput drug screen, we found that tenovin-1 was antiviral against the flaviviruses Zika virus and dengue virus. Tenovin-1 is a sirtuin inhibitor, and here we found that inhibition of sirtuins, but not inhibition of the related histone deacetylases, is potently antiviral against diverse arboviruses. Sirtuin inhibitors block infection of arboviruses in multiple human cell types. We found that sirtuin inhibitors arrest infection downstream of entry but that they do so at an early step, preventing the accumulation of viral RNA and protein. However, sirtuin inhibitors had no impact on the replication of flaviviral replicons, suggesting a defect in the establishment of replication. Consistent with this, we found that sirtuin inhibitors impacted double-stranded RNA (dsRNA) accumulation during flaviviral infection. Since these viruses infect vector insects, we also tested whether sirtuin inhibitors impacted infection of adult flies and found that these inhibitors blocked infection; therefore, they target highly conserved facets of replication. Taken together, these results suggest that sirtuin inhibitors represent a new class of potent host-targeting antivirals.IMPORTANCE Arthropod-borne viruses are diverse pathogens and are associated with human disease. Through high-throughput drug screening, we found that sirtuin inhibitors are potently antiviral against diverse arboviruses, including flaviviruses such as West Nile virus, bunyaviruses such as Rift Valley fever virus, and alphaviruses such as chikungunya virus. Sirtuin inhibitors block infection of these viruses in multiple human cell types. Moreover, we found that sirtuin inhibitors arrest infection downstream of entry but that they do so at an early step, preventing the accumulation of viral RNA and protein. Since these viruses infect vector insects, we also tested whether sirtuin inhibitors impacted infection of adult flies and found that these inhibitors blocked infection; therefore, they target highly conserved facets of replication. Taken together, these results suggest that sirtuin inhibitors represent a new class of potent host-targeting antivirals.
Keywords: alphavirus; arbovirus; bunyavirus; flavivirus; sirtinol; sirtuin.
Copyright © 2019 Hackett et al.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
