Mitochondrial Akt Signaling Modulated Reprogramming of Somatic Cells
- PMID: 31289326
- PMCID: PMC6616364
- DOI: 10.1038/s41598-019-46359-6
Mitochondrial Akt Signaling Modulated Reprogramming of Somatic Cells
Abstract
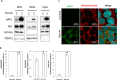
The signaling mechanisms controlling somatic cell reprogramming are not fully understood. In this study, we report a novel role for mitochondrial Akt1 signaling that enhanced somatic cell reprogramming efficiency. The role of mitochondrial Akt1 in somatic cell reprogramming was investigated by transducing fibroblasts with the four reprogramming factors (Oct4, Sox2, Klf4, c-Myc) in conjunction with Mito-Akt1, Mito-dnAkt1, or control virus. Mito-Akt1 enhanced reprogramming efficiency whereas Mito-dnAkt1 inhibited reprogramming. The resulting iPSCs formed embryoid bodies in vitro and teratomas in vivo. Moreover, Oct4 and Nanog promoter methylation was reduced in the iPSCs generated in the presence of Mito-Akt1. Akt1 was activated and translocated into mitochondria after growth factor stimulation in embryonic stem cells (ESCs). To study the effect of mitochondrial Akt in ESCs, a mitochondria-targeting constitutively active Akt1 (Mito-Akt1) was expressed in ESCs. Gene expression profiling showed upregulation of genes that promote stem cell proliferation and survival and down-regulation of genes that promote differentiation. Analysis of cellular respiration indicated similar metabolic profile in the resulting iPSCs and ESCs, suggesting comparable bioenergetics. These findings showed that activation of mitochondrial Akt1 signaling was required during somatic cell reprogramming.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

