Fucoidan inhibits epithelial-to-mesenchymal transition via regulation of the HIF-1α pathway in mammary cancer cells under hypoxia
- PMID: 31289504
- PMCID: PMC6539587
- DOI: 10.3892/ol.2019.10283
Fucoidan inhibits epithelial-to-mesenchymal transition via regulation of the HIF-1α pathway in mammary cancer cells under hypoxia
Abstract
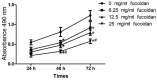
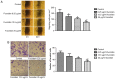
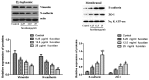
This study examined the effects of fucoidan on epithelial-to-mesenchymal transition (EMT) in a human triple-negative breast cancer (TNBC) cell line in a hypoxic microenvironment. Transwell and wound-healing assays were performed to analyze the invasion and migration of MDA-MB-231 human mammary cancer cells, respectively. The expression levels of EMT markers and hypoxia-inducible factor-1α (HIF-1α) were detected through western blotting. Under hypoxia, fucoidan treatment inhibited proliferation of breast cancer cells. Fucoidan also suppressed the invasion and migration of MDA-MB-231 cells. Western blotting revealed that fucoidan treatment significantly reduced the protein expression levels of HIF-1α and HIF-1 target genes. Furthermore, the nuclear translocation and activity of HIF-1α were reduced. Fucoidan treatment significantly downregulated the expression levels of mesenchymal markers (N-cadherin and vimentin), but upregulated the expression levels of the epithelial markers zonula occludens-1 and E-cadherin. In addition, overexpression of HIF1-α protected cells from fucoidan-mediated suppression of migration and invasion. These data suggested that fucoidan may inhibit EMT in human TNBC cells via downregulation of the HIF1-α signaling pathway.
Keywords: epithelial-to-mesenchymal transition; fucoidan; hypoxia-inducible factor-1α; triple-negative breast cancer.
Figures


Similar articles
-
Hypoxia-inducible factor 1α-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis.Hum Reprod. 2016 Jun;31(6):1327-38. doi: 10.1093/humrep/dew081. Epub 2016 Apr 19. Hum Reprod. 2016. PMID: 27094478
-
Gli-1 is crucial for hypoxia-induced epithelial-mesenchymal transition and invasion of breast cancer.Tumour Biol. 2015 Apr;36(4):3119-26. doi: 10.1007/s13277-014-2948-z. Epub 2014 Dec 14. Tumour Biol. 2015. PMID: 25501705
-
Coenzyme Q0 defeats NLRP3-mediated inflammation, EMT/metastasis, and Warburg effects by inhibiting HIF-1α expression in human triple-negative breast cancer cells.Arch Toxicol. 2023 Apr;97(4):1047-1068. doi: 10.1007/s00204-023-03456-w. Epub 2023 Feb 27. Arch Toxicol. 2023. PMID: 36847822
-
GLI1 is involved in HIF-1α-induced migration, invasion, and epithelial-mesenchymal transition in glioma cells.Folia Histochem Cytobiol. 2022;60(2):156-166. doi: 10.5603/FHC.a2022.0014. Epub 2022 May 23. Folia Histochem Cytobiol. 2022. PMID: 35603730
-
Hypoxia Induces Epithelial-Mesenchymal Transition in Follicular Thyroid Cancer: Involvement of Regulation of Twist by Hypoxia Inducible Factor-1α.Yonsei Med J. 2015 Nov;56(6):1503-14. doi: 10.3349/ymj.2015.56.6.1503. Yonsei Med J. 2015. PMID: 26446630 Free PMC article.
Cited by
-
Bioinformatics Analysis and Verification of Metabolic Abnormalities in Esophageal Squamous Carcinoma.Comb Chem High Throughput Screen. 2024;27(2):273-283. doi: 10.2174/1386207326666230331083724. Comb Chem High Throughput Screen. 2024. PMID: 37005515
-
Effect, sensitivity, specificity and accuracy of ultrasonic assessment of axillary lymph node-negative breast cancer.Pak J Med Sci. 2023 Sep-Oct;39(5):1366-1371. doi: 10.12669/pjms.39.5.7260. Pak J Med Sci. 2023. PMID: 37680794 Free PMC article.
-
Fucoidan and Lung Function: Value in Viral Infection.Mar Drugs. 2020 Dec 24;19(1):4. doi: 10.3390/md19010004. Mar Drugs. 2020. PMID: 33374149 Free PMC article. Review.
-
A Review of Natural Therapies Potentially Relevant in Triple Negative Breast Cancer Aimed at Targeting Cancer Cell Vulnerabilities.Integr Cancer Ther. 2020 Jan-Dec;19:1534735420975861. doi: 10.1177/1534735420975861. Integr Cancer Ther. 2020. PMID: 33243021 Free PMC article. Review.
-
Functions and mechanisms of RNA m6A regulators in breast cancer (Review).Int J Oncol. 2024 Sep;65(3):86. doi: 10.3892/ijo.2024.5674. Epub 2024 Jul 26. Int J Oncol. 2024. PMID: 39054967 Free PMC article. Review.
References
-
- Lester RD, Jo M, Campana WM, Gonias SL. Erythropoietin promotes MCF-7 breast cancer cell migration by an ERK/mitogen-activated protein kinase-dependent pathway and is primarily responsible for the increase in migration observed in hypoxia. J Biol Chem. 2005;280:39273–39277. doi: 10.1074/jbc.M509446200. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
