Chidamide, a histone deacetylase inhibitor, induces growth arrest and apoptosis in multiple myeloma cells in a caspase-dependent manner
- PMID: 31289512
- PMCID: PMC6540238
- DOI: 10.3892/ol.2019.10301
Chidamide, a histone deacetylase inhibitor, induces growth arrest and apoptosis in multiple myeloma cells in a caspase-dependent manner
Abstract
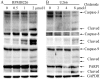
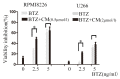
Chidamide, a novel histone deacetylase (HDAC) inhibitor, induces antitumor effects in various types of cancer. The present study aimed to evaluate the cytotoxic effect of chidamide on multiple myeloma and the underlying mechanisms involved. Viability of multiple myeloma cells upon chidamide treatment was determined by the Cell Counting Kit-8 assay. Apoptosis induction and cell cycle alteration were detected by flow cytometry. Specific apoptosis-associated proteins and cell cycle proteins were evaluated by western blot analysis. Chidamide suppressed cell viability in a time- and dose-dependent manner. Chidamide treatment markedly suppressed the expression of type I HDACs and further induced the acetylation of histones H3 and H4. In addition, it promoted G0/G1 arrest by decreasing cyclin D1 and c-myc expression, and increasing phosphorylated-cellular tumor antigen p53 and cyclin-dependent kinase inhibitor 1 (p21) expression in a dose-dependent manner. Treatment with chidamide induced cell apoptosis by upregulating the apoptosis regulator Bax/B-cell lymphoma 2 ratio in a caspase-dependent manner. In addition, the combination of chidamide with bortezomib, a proteasome inhibitor widely used as a therapeutic agent for multiple myeloma, resulted in enhanced inhibition of cell viability. In conclusion, chidamide induces a marked antimyeloma effect by inducing G0/G1 arrest and apoptosis via a caspase-dependent pathway. The present study provides evidence for the clinical application of chidamide in multiple myeloma.
Keywords: G0/G1 arrest; apoptosis; cell cycle; chidamide; histone deacetylase inhibitors; multiple myeloma.
Figures
Similar articles
-
Chidamide, a histone deacetylase inhibitor, functions as a tumor inhibitor by modulating the ratio of Bax/Bcl-2 and P21 in pancreatic cancer.Oncol Rep. 2015 Jan;33(1):304-10. doi: 10.3892/or.2014.3595. Epub 2014 Nov 10. Oncol Rep. 2015. PMID: 25384499
-
Chidamide and 5-flurouracil show a synergistic antitumor effect on human colon cancer xenografts in nude mice.Neoplasma. 2016;63(2):193-200. doi: 10.4149/203_150422N214. Neoplasma. 2016. PMID: 26774139
-
[Inducing effect of chidamide on apoptosis of multiple myeloma cells and its relerance to DNA damage response].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015 Apr;23(2):450-4. doi: 10.7534/j.issn.1009-2137.2015.02.030. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015. PMID: 25948203 Chinese.
-
Chidamide: Targeting epigenetic regulation in the treatment of hematological malignancy.Hematol Oncol. 2023 Aug;41(3):301-309. doi: 10.1002/hon.3088. Epub 2022 Oct 25. Hematol Oncol. 2023. PMID: 36251458 Review.
-
Development of chidamide for peripheral T-cell lymphoma, the first orphan drug approved in China.Intractable Rare Dis Res. 2016 Aug;5(3):185-91. doi: 10.5582/irdr.2016.01024. Intractable Rare Dis Res. 2016. PMID: 27672541 Free PMC article. Review.
Cited by
-
Histone deacetylase inhibitors for leukemia treatment: current status and future directions.Eur J Med Res. 2024 Oct 26;29(1):514. doi: 10.1186/s40001-024-02108-8. Eur J Med Res. 2024. PMID: 39456044 Free PMC article. Review.
-
Chidamide acts on the histone deacetylase-mediated miR-34a/Bcl-2 axis to regulate NB4 cell line proliferation and apoptosis.Kaohsiung J Med Sci. 2020 Dec;36(12):1004-1013. doi: 10.1002/kjm2.12283. Epub 2020 Aug 12. Kaohsiung J Med Sci. 2020. PMID: 32783381 Free PMC article.
-
Venetoclax in combination with chidamide and dexamethasone in relapsed/refractory primary plasma cell leukemia without t(11;14): A case report.World J Clin Cases. 2021 Feb 16;9(5):1175-1183. doi: 10.12998/wjcc.v9.i5.1175. World J Clin Cases. 2021. PMID: 33644182 Free PMC article.
-
Identification of potential target genes of breast cancer in response to Chidamide treatment.Front Mol Biosci. 2022 Nov 8;9:999582. doi: 10.3389/fmolb.2022.999582. eCollection 2022. Front Mol Biosci. 2022. PMID: 36425653 Free PMC article.
-
The combination of brentuximab vedotin and chidamide synergistically suppresses the proliferation of T-cell lymphoma cells through the enhancement of apoptosis.Cancer Chemother Pharmacol. 2024 Feb;93(2):137-149. doi: 10.1007/s00280-023-04609-5. Epub 2023 Nov 3. Cancer Chemother Pharmacol. 2024. PMID: 37921901 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
