Detecting predictive androgen receptor modifications in circulating prostate cancer cells
- PMID: 31289619
- PMCID: PMC6609250
- DOI: 10.18632/oncotarget.3925
Detecting predictive androgen receptor modifications in circulating prostate cancer cells
Abstract
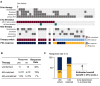
Molecular modifications of the androgen receptor (AR) can cause resistance to androgen deprivation therapy (ADT) in prostate cancer patients. Since lack of representative tumor samples hinders therapy adjustments according to emerging AR-modifications, we evaluated simultaneous detection of the two most common AR modifications (AR-V7 splice variant and AR point mutations) in circulating tumor cells (CTCs). We devised a single-tube assay to detect AR-V7 splice variants and AR point mutations in CTCs using immunomagnetic cell isolation, followed by quantitative real-time PCR and DNA pyrosequencing. We prospectively investigated 47 patients with PSA progression awaiting therapy switch. Comparison of response to newly administered therapy and CTC-AR-status allowed effect size estimation. Nineteen (51%) of 37 patients with detectable CTCs carried AR-modifications. Seventeen patients carried the AR-V7 splice variant, one harbored a p.T878A point mutation and one harbored both AR-V7 and a p.H875Y mutation. We estimated a positive predictive value for response and non-response to therapy by AR status in CTCs of ~94%. Based on a conservative calculation, we estimated the effect size for molecularly-informed therapy switches for prospective clinical trial planning to ~27%. In summary, the ability to determine key resistance-mediating AR modifications in CTCs has the potential to considerably improve prostate cancer treatment.
Keywords: androgen receptor modification; castration-resistant prostate cancer; circulating tumor cells; splice variants.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have declared that no conflict of interest exists.
Figures

References
-
- Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. - DOI - PMC - PubMed
-
- Al Nakouzi N, Le Moulec S, Albiges L, Wang C, Beuzeboc P, Gross-Goupil M, de La Motte Rouge T, Guillot A, Gajda D, Massard C, et al. Cabazitaxel Remains Active in Patients Progressing After Docetaxel Followed by Novel Androgen Receptor Pathway Targeted Therapies. European urology. 2015;68:228–35. doi: 10.1016/j.eururo.2014.04.015. - DOI - PubMed
-
- Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, Gumerlock PH, deVere White RW, Pretlow TG, Harris SE, et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11:450–459. doi: 10.1210/mend.11.4.9906. - DOI - PubMed
-
- Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, Miyamoto M. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Research. 2003;63:149–153. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

