Regulation of platelet-activating factor-mediated interleukin-6 promoter activation by the 48 kDa but not the 45 kDa isoform of protein tyrosine phosphatase non-receptor type 2
- PMID: 31289638
- PMCID: PMC6593612
- DOI: 10.1186/s13578-019-0316-9
Regulation of platelet-activating factor-mediated interleukin-6 promoter activation by the 48 kDa but not the 45 kDa isoform of protein tyrosine phosphatase non-receptor type 2
Abstract
Background: An underlying state of inflammation is thought to be an important cause of cardiovascular disease. Among cells involved in the early steps of atherosclerosis, monocyte-derived dendritic cells (Mo-DCs) respond to inflammatory stimuli, including platelet-activating factor (PAF), by the induction of various cytokines, such as interleukin 6 (IL-6). PAF is a potent phospholipid mediator involved in both the onset and progression of atherosclerosis. It mediates its effects by binding to its cognate G-protein coupled receptor, PAFR. Activation of PAFR-induced signaling pathways is tightly coordinated to ensure specific cell responses.
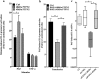
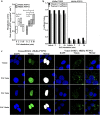
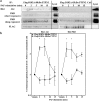
Results: Here, we report that PAF stimulated the phosphatase activity of both the 45 and 48 kDa isoforms of the protein tyrosine phosphatase non-receptor type 2 (PTPN2). However, we found that only the 48 kDa PTPN2 isoform has a role in PAFR-induced signal transduction, leading to activation of the IL-6 promoter. In luciferase reporter assays, expression of the 48 kDa, but not the 45 kDa, PTPN2 isoform increased human IL-6 (hIL-6) promoter activity by 40% after PAF stimulation of HEK-293 cells, stably transfected with PAFR (HEK-PAFR). Our results suggest that the differential localization of the PTPN2 isoforms and the differences in PAF-induced phosphatase activation may contribute to the divergent modulation of PAF-induced IL-6 promoter activation. The involvement of PTPN2 in PAF-induced IL-6 expression was confirmed in immature Mo-DCs (iMo-DCs), using siRNAs targeting the two isoforms of PTPN2, where siRNAs against the 48 kDa PTPN2 significantly inhibited PAF-stimulated IL-6 mRNA expression. Pharmacological inhibition of several signaling pathways suggested a role for PTPN2 in early signaling events. Results obtained by Western blot confirmed that PTPN2 increased the activation of the PI3K/Akt pathway via the modulation of protein kinase D (PKD) activity. WT PKD expression counteracted the effect of PTPN2 on PAF-induced IL-6 promoter transactivation and phosphorylation of Akt. Using siRNAs targeting the individual isoforms of PTPN2, we confirmed that these pathways were also active in iMo-DCs.
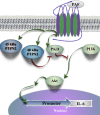
Conclusion: Taken together, our data suggest that PTPN2, in an isoform-specific manner, could be involved in the positive regulation of PI3K/Akt activation, via the modulation of PKD activity, allowing for the maximal induction of PAF-stimulated IL-6 mRNA expression.
Keywords: GPCR; IL-6; PTPN2; Platelet-activating factor; Protein tyrosine phosphatase; TC-PTP.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures






References
-
- Demopoulos CA, Karantonis HC, Antonopoulou S. Platelet activating factor—a molecular link between atherosclerosis theories. Eur J Lipid Sci Technol. 2003;105(11):705–716. doi: 10.1002/ejlt.200300845. - DOI
-
- Brown SL, Jala VR, Raghuwanshi SK, Nasser MW, Haribabu B, Richardson RM. Activation and regulation of platelet-activating factor receptor: role of G(i) and G(q) in receptor-mediated chemotactic, cytotoxic, and cross-regulatory signals. J Immunol Baltim Md 1950. 2006;177(5):3242–3249. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

