Liver Bioengineering: Promise, Pitfalls, and Hurdles to Overcome
- PMID: 31289714
- PMCID: PMC6615568
- DOI: 10.1007/s40472-019-00236-3
Liver Bioengineering: Promise, Pitfalls, and Hurdles to Overcome
Abstract
Purpose of review: In this review, we discuss the recent advancements in liver bioengineering and cell therapy and future advancements to improve the field towards clinical applications.
Recent findings: 3D printing, hydrogel-based tissue fabrication, and the use of native decellularized liver extracellular matrix as a scaffold are used to develop whole or partial liver substitutes. The current focus is on developing a functional liver graft through achieving a non-leaky endothelium and a fully constructed bile duct. Use of cell therapy as a treatment is less invasive and less costly compared to transplantation, however, lack of readily available cell sources with low or no immunogenicity and contradicting outcomes of clinical trials are yet to be overcome.
Summary: Liver bioengineering is advancing rapidly through the development of in vitro and in vivo tissue and organ models. Although there are major challenges to overcome, through optimization of the current methods and successful integration of induced pluripotent stem cells, the development of readily available, patient-specific liver substitutes can be achieved.
Keywords: Cell therapy; Decellularization; Induced pluripotent stem cells; Liver; Tissue engineering.
Conflict of interest statement
Conflict of Interest Basak Uygun reports grants from National Institutes of Health (R01DK084053) and has a financial interest in Organ Solutions, LLC (reviewed and arranged by MGH and Partners HealthCare in accordance with their conflict of interest policies). Aylin Acun and Ruben Oganesyan declare no conflict of interest.
Figures
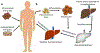
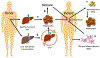
References
-
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. - PubMed
-
- Du Y, et al. Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab Chip. 2017;17:782–94. - PubMed
-
- Mi S, Yi X, Du Z, Xu Y, Sun W. Construction of a liver sinusoid based on the laminar flow on chip and self-assembly of endothelial cells. Biofabrication. 2018;10:025010. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
