A Dual Targeting Magnetic Nanoparticle for Human Cancer Detection
- PMID: 31289961
- PMCID: PMC6616609
- DOI: 10.1186/s11671-019-3049-0
A Dual Targeting Magnetic Nanoparticle for Human Cancer Detection
Abstract
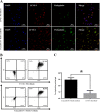
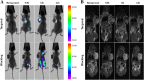
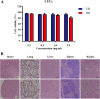
Malignant tumors are a major threat to human life and high lymphatic vessel density is often associated with metastatic tumors. With the discovery of molecules targeted at the lymphatic system such as lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) and Podoplanin, many studies have been performed to determine the role of lymphatic endothelial cells (LECs) in tumor metastasis. However, disadvantages such as non-specificity and high cost limit their research and diagnostic applications. In this study, Fe3O4@KCTS, a core-shell type of magnetic nanoparticles, was prepared by activating Fe3O4 with carbodiimide and cross-linking it with α-ketoglutarate chitosan (KCTS). The LYVE-1 and Podoplanin antibodies were then incorporated onto the surface of these magnetic nanoparticles and as a result, dual-targeting magnetic nanoprobes were developed. The experimental tests of this study demonstrated that a dual-targeting magnetic nanoprobe with high-purity LECs from tumor tissues was successfully developed, providing a basis for clinical application of LECs in colorectal cancer treatment as well as in early clinical diagnosis using bimodal imaging.
Keywords: LYVE-1; Lymphatic endothelial cells; Magnetic nanoparticles; Podoplanin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Onder L, Morbe U, Pikor N, Novkovic M, Cheng HW, Hehlgans T, Pfeffer K, Becher B, Waisman A, Rulicke T, gommerman J, Mueller CG, Sawa S, Scandella E, Ludewig B. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity. 2017;47:80–92.e4. doi: 10.1016/j.immuni.2017.05.008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

