Artemisinin-(Iso)quinoline Hybrids by C-H Activation and Click Chemistry: Combating Multidrug-Resistant Malaria
- PMID: 31290221
- PMCID: PMC6899722
- DOI: 10.1002/anie.201907224
Artemisinin-(Iso)quinoline Hybrids by C-H Activation and Click Chemistry: Combating Multidrug-Resistant Malaria
Abstract
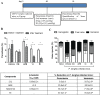
A substantial challenge worldwide is emergent drug resistance in malaria parasites against approved drugs, such as chloroquine (CQ). To address these unsolved CQ resistance issues, only rare examples of artemisinin (ART)-based hybrids have been reported. Moreover, protein targets of such hybrids have not been identified yet, and the reason for the superior efficacy of these hybrids is still not known. Herein, we report the synthesis of novel ART-isoquinoline and ART-quinoline hybrids showing highly improved potencies against CQ-resistant and multidrug-resistant P. falciparum strains (EC50 (Dd2) down to 1.0 nm; EC50 (K1) down to 0.78 nm) compared to CQ (EC50 (Dd2)=165.3 nm; EC50 (K1)=302.8 nm) and strongly suppressing parasitemia in experimental malaria. These new compounds are easily accessible by step-economic C-H activation and copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) click reactions. Through chemical proteomics, putatively hybrid-binding protein targets of the ART-quinolines were successfully identified in addition to known targets of quinoline and artemisinin alone, suggesting that the hybrids act through multiple modes of action to overcome resistance.
Keywords: antimalarial agents; artemisinin; drug resistance; hybridization; proteomics.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- World Health Organization (2018), World Malaria Report 2018; http://www.who.int/iris/handle/10665/275867. License: CC BY-NC-SA 3.0 IGO.
-
- Bray P., Ward S., O'Neill P. in Malaria: drugs, disease and post-genomic biology, Springer, Berlin, 2005, pp. 3–38.
-
- None
-
- Tu Y., Nat. Med. 2011, 17, 1217–1220; - PubMed
-
- Tu Y., Angew. Chem. Int. Ed. 2016, 55, 10210–10226; - PubMed
- Angew. Chem. 2016, 128, 10366–10382.
Publication types
MeSH terms
Substances
Grants and funding
- TS 87/16-3/Deutsche Forschungsgemeinschaft/International
- Alexander von Humboldt-Stiftung/International
- Friedrich-Alexander-Universität Erlangen-Nürnberg (DE), Emerging Fields Initiative (EFI) "Chemistry in Live Cells"/International
- APP0088/2016/Fundação de Amparo à Pesquisa do Estado da Bahia/International
- 1642178247/Fiocruz/Inova/International
LinkOut - more resources
Full Text Sources
Medical

