Matrine promotes neural circuit remodeling to regulate motor function in a mouse model of chronic spinal cord injury
- PMID: 31290454
- PMCID: PMC6676875
- DOI: 10.4103/1673-5374.259625
Matrine promotes neural circuit remodeling to regulate motor function in a mouse model of chronic spinal cord injury
Abstract
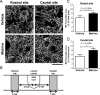
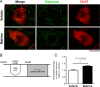
In chronic phase of spinal cord injury, functional recovery is more untreatable compared with early intervention in acute phase of spinal cord injury. In the last decade, several combination therapies successfully improved motor dysfunction in chronic spinal cord injury. However, their effectiveness is not sufficient. We previously found a new effective compound for spinal cord injury, matrine, which induced axonal growth and functional recovery in acute spinal cord injury mice via direct activation of extracellular heat shock protein 90. Although our previous study clarified that matrine was an activator of extracellular heat shock protein 90, the potential of matrine for spinal cord injury in chronic phase has not been sufficiently evaluated. Thus, this study aimed to investigate whether matrine ameliorates chronic spinal cord injury in mice. Once daily intragastric administration of matrine (100 μmol/kg per day) to spinal cord injury mice were starte at 28 days after injury, and continued for 154 days. Continuous matrine treatment improved hindlimb motor function in chronic spinal cord injury mice. In injured spinal cords of the matrine-treated mice, the density of neurofilament-H-positive axons was increased. Moreover, matrine treatment increased the density of bassoon-positive presynapses in contact with choline acetyltransferase-positive motor neurons in the lumbar spinal cord. These findings suggest that matrine promotes remodeling and reconnection of neural circuits to regulate hindlimb movement. All protocols were approved by the Committee for Animal Care and Use of the Sugitani Campus of the University of Toyama (approval No. A2013INM-1 and A2016INM-3) on May 7, 2013 and May 17, 2016, respectively.
Keywords: Basso Mouse Scale; Body Support Score; Sophora flavescens; axonal growth; chronic spinal cord injury; hindlimb locomotor; immunohistochemistry; matrine; presynapse; synaptogenesis.
Conflict of interest statement
None
Figures




Similar articles
-
Matrine Directly Activates Extracellular Heat Shock Protein 90, Resulting in Axonal Growth and Functional Recovery in Spinal Cord Injured-Mice.Front Pharmacol. 2018 May 7;9:446. doi: 10.3389/fphar.2018.00446. eCollection 2018. Front Pharmacol. 2018. PMID: 29867458 Free PMC article.
-
The Extract of Roots of Sophora flavescens Enhances the Recovery of Motor Function by Axonal Growth in Mice with a Spinal Cord Injury.Front Pharmacol. 2016 Jan 14;6:326. doi: 10.3389/fphar.2015.00326. eCollection 2015. Front Pharmacol. 2016. PMID: 26834638 Free PMC article.
-
Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury.J Neurosurg Spine. 2016 Dec;25(6):745-755. doi: 10.3171/2016.4.SPINE15923. Epub 2016 Jul 1. J Neurosurg Spine. 2016. PMID: 27367940
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
-
From cortex to cord: motor circuit plasticity after spinal cord injury.Neural Regen Res. 2019 Dec;14(12):2054-2062. doi: 10.4103/1673-5374.262572. Neural Regen Res. 2019. PMID: 31397332 Free PMC article. Review.
Cited by
-
Multi-target neural circuit reconstruction and enhancement in spinal cord injury.Neural Regen Res. 2026 Mar 1;21(3):957-971. doi: 10.4103/NRR.NRR-D-24-00434. Epub 2025 Jan 29. Neural Regen Res. 2026. PMID: 39885668 Free PMC article.
-
Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Promote the Recovery of Spinal Cord Injury and Inhibit Ferroptosis by Inactivating IL-17 Pathway.J Mol Neurosci. 2024 Mar 27;74(2):33. doi: 10.1007/s12031-024-02209-3. J Mol Neurosci. 2024. PMID: 38536541
-
PLG Bridge Implantation in Chronic SCI Promotes Axonal Elongation and Myelination.ACS Biomater Sci Eng. 2019 Dec 9;5(12):6679-6690. doi: 10.1021/acsbiomaterials.9b01012. Epub 2019 Nov 14. ACS Biomater Sci Eng. 2019. PMID: 33423486 Free PMC article.
-
Research progress on the pharmacological effects of matrine.Front Neurosci. 2022 Aug 23;16:977374. doi: 10.3389/fnins.2022.977374. eCollection 2022. Front Neurosci. 2022. PMID: 36110092 Free PMC article. Review.
-
miR-672-3p Promotes Functional Recovery in Rats with Contusive Spinal Cord Injury by Inhibiting Ferroptosis Suppressor Protein 1.Oxid Med Cell Longev. 2022 Feb 21;2022:6041612. doi: 10.1155/2022/6041612. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35237382 Free PMC article.
References
-
- Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol. 2004;91:1832–1839. - PubMed
-
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
-
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

