Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined With a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1)
- PMID: 31290965
- PMCID: PMC6822141
- DOI: 10.1093/ijnp/pyz039
Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined With a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1)
Abstract
Background: About one-third of patients with depression fail to achieve remission despite treatment with multiple antidepressants and are considered to have treatment-resistant depression.
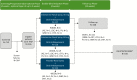
Methods: This Phase 3, double-blind, multicenter study enrolled adults with moderate-to-severe depression and nonresponse to ≥2 antidepressants in the current depression episode. Eligible patients (N = 346) were randomized (1:1:1) to twice-weekly nasal spray treatment (esketamine [56 or 84 mg] or placebo) plus a newly initiated, open-label, oral antidepressant taken daily for 4 weeks. The primary efficacy endpoint was change from baseline to day 28 in the Montgomery-Asberg Depression Rating Scale total score, performed by blinded, remote raters. Based on the predefined statistical testing sequence, esketamine 84 mg/antidepressant had to be significant for esketamine 56 mg/antidepressant to be formally tested.
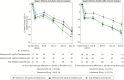
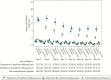
Results: Statistical significance was not achieved with esketamine 84 mg/antidepressant compared with antidepressant/placebo (least squares [LS] means difference [95% CI]: -3.2 [-6.88, 0.45]; 2-sided P value = .088). Although esketamine 56 mg/antidepressant could not be formally tested, the LS means difference was -4.1 [-7.67, -0.49] (nominal 2-sided P value = .027). The most common (>20%) adverse events reported for esketamine/antidepressant were nausea, dissociation, dizziness, vertigo, and headache.
Conclusions: Statistical significance was not achieved for the primary endpoint; nevertheless, the treatment effect (Montgomery-Asberg Depression Rating Scale) for both esketamine/antidepressant groups exceeded what has been considered clinically meaningful for approved antidepressants vs placebo. Safety was similar between esketamine/antidepressant groups and no new dose-related safety concerns were identified. This study provides supportive evidence for the safety and efficacy of esketamine nasal spray as a new, rapid-acting antidepressant for patients with treatment-resistant depression.
Trial registration: ClinicalTrials.gov identifier: NCT02417064.
Keywords: esketamine; ketamine; s-ketamine; treatment-resistant depression.
© The Author(s) 2019. Published by Oxford University Press on behalf of CINP.
Figures



References
-
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5). 5th ed.Washington, DC: American Psychiatric Association.
-
- Baldessarini RJ, Forte A, Selle V, Sim K, Tondo L, Undurraga J, Vázquez GH (2017) Morbidity in depressive disorders. Psychother Psychosom 86:65–72. - PubMed
-
- Bauer M, Severus E, Köhler S, Whybrow PC, Angst J, Möller HJ; Wfsbp Task Force on Treatment Guidelines for Unipolar Depressive Disorders (2015) World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. Part 2: maintenance treatment of major depressive disorder-update 2015. World J Biol Psychiatry 16:76–95. - PubMed
-
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM (1998) Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). J Trauma Stress 11:125–136. - PubMed
-
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, Hough D, Sanacora G, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 175:620–630. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

