Innate CD8αα+ cells promote ILC1-like intraepithelial lymphocyte homeostasis and intestinal inflammation
- PMID: 31291255
- PMCID: PMC6619599
- DOI: 10.1371/journal.pone.0215883
Innate CD8αα+ cells promote ILC1-like intraepithelial lymphocyte homeostasis and intestinal inflammation
Abstract
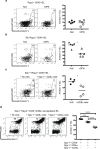
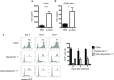
Innate CD8αα+ cells, also referred to as iCD8α cells, are TCR-negative intraepithelial lymphocytes (IEL) possessing cytokine and chemokine profiles and functions related to innate immune cells. iCD8α cells constitute an important source of osteopontin in the intestinal epithelium. Osteopontin is a pleiotropic cytokine with diverse roles in bone and tissue remodeling, but also has relevant functions in the homeostasis of immune cells. In this report, we present evidence for the role of iCD8α cells in the homeostasis of TCR-negative NKp46+NK1.1+ IEL (ILC1-like). We also show that the effect of iCD8α cells on ILC1-like IEL is enhanced in vitro by osteopontin. We show that in the absence of iCD8α cells, the number of NKp46+NK1.1+ IEL is significantly reduced. These ILC1-like cells are involved in intestinal pathogenesis in the anti-CD40 mouse model of intestinal inflammation. Reduced iCD8α cell numbers results in a milder form of intestinal inflammation in this disease model, whereas treatment with osteopontin increases disease severity. Collectively, our results suggest that iCD8α cells promote survival of NKp46+NK1.1+ IEL, which significantly impacts the development of intestinal inflammation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Das G, Augustine MM, Das J, Bottomly K, Ray P, Ray A. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci U S A. 2003;100(9):5324–9. PMC1535703. 10.1073/pnas.0831037100 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

