Improving medication adherence and outcomes in adult kidney transplant patients using a personal systems approach: SystemCHANGE™ results of the MAGIC randomized clinical trial
- PMID: 31291507
- PMCID: PMC7179766
- DOI: 10.1111/ajt.15528
Improving medication adherence and outcomes in adult kidney transplant patients using a personal systems approach: SystemCHANGE™ results of the MAGIC randomized clinical trial
Abstract
This study determined if a SystemCHANGE™ intervention was more efficacious than attention control in increasing immunosuppressive medication adherence and improving outcomes in adult kidney transplant recipients during a 6-month intervention phase and subsequent 6-month (no intervention) maintenance phase. The SystemCHANGE™ intervention taught patients to use person-level quality improvement strategies to link adherence to established daily routines, environmental cues, and supportive people. Eighty-nine patients (average age 51.8 years, 58% male, 61% African American) completed the 6-month intervention phase. Using an intent-to-treat analysis, at 6 months, medication adherence for SystemCHANGE™ (median 0.91, IQR 0.76-0.96) and attention control (median 0.67, IQR 0.52-0.72) patients differed markedly (difference in medians 0.24, 95% CI 0.13-0.30, P < .001). At the conclusion of the subsequent 6-month maintenance phase, the gap between medication adherence for SystemCHANGE™ (median 0.77, IQR 0.56-0.94) and attention control (median 0.60, IQR 0.44-0.73) patients remained large (difference in medians 0.17, 95% CI 0.06-0.33, P = .004). SystemCHANGE™ patients evidenced lower mean creatinine and BUN at 12 months and more infections at 6 and 12 months. This first fully powered RCT testing SystemCHANGE™ to improve and maintain medication adherence in kidney transplant recipients demonstrated large, clinically meaningful improvements in medication adherence. Clinical Trial Registration: NCT02416479.
Keywords: clinical research/practice; clinical trial; health services and outcomes research; immunosuppressant; immunosuppression/immune modulation; kidney transplantation/nephrology.
© 2019 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures
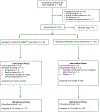
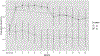

Comment in
-
Multicomponent interventions improve adherence-Where do we go from here?Am J Transplant. 2020 Jan;20(1):5-6. doi: 10.1111/ajt.15632. Epub 2019 Oct 30. Am J Transplant. 2020. PMID: 31587438 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

