Hepatic Forkhead Box Protein A3 Regulates ApoA-I (Apolipoprotein A-I) Expression, Cholesterol Efflux, and Atherogenesis
- PMID: 31291759
- PMCID: PMC6656627
- DOI: 10.1161/ATVBAHA.119.312610
Hepatic Forkhead Box Protein A3 Regulates ApoA-I (Apolipoprotein A-I) Expression, Cholesterol Efflux, and Atherogenesis
Abstract
Objective: To determine the role of hepatic FOXA3 (forkhead box A3) in lipid metabolism and atherosclerosis. Approach and Results: Hepatic FOXA3 expression was reduced in diabetic or high fat diet-fed mice or patients with nonalcoholic steatohepatitis. We then used adenoviruses to overexpress or knock down hepatic FOXA3 expression. Overexpression of FOXA3 in the liver increased hepatic ApoA-I (apolipoprotein A-I) expression, plasma HDL-C (high-density lipoprotein cholesterol) level, macrophage cholesterol efflux, and macrophage reverse cholesterol transport. In contrast, knockdown of hepatic FOXA3 expression had opposite effects. We further showed that FOXA3 directly bound to the promoter of the Apoa1 gene to regulate its transcription. Finally, AAV8 (adeno-associated virus serotype 8)-mediated overexpression of human FOXA3 in the hepatocytes of Apoe-/- (apolipoprotein E-deficient) mice raised plasma HDL-C levels and significantly reduced atherosclerotic lesions.
Conclusions: Hepatocyte FOXA3 protects against atherosclerosis by inducing ApoA-I and macrophage reverse cholesterol transport.
Keywords: FOXA3; atherosclerosis; cholesterol efflux; liver; macrophages.
Figures





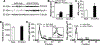
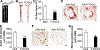
References
-
- Yamashita S, Sakai N, Hirano K, Ishigami M, Maruyama T, Nakajima N, Matsuzawa Y. Roles of plasma lipid transfer proteins in reverse cholesterol transport. Front Biosci. 2001;6:D366–387 - PubMed
-
- Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D’Agostino RB Sr., Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M, Rader DJ. High-density lipoproteins: A consensus statement from the national lipid association. J Clin Lipidol. 2013;7:484–525 - PubMed
-
- Lee-Rueckert M, Escola-Gil JC, Kovanen PT. Hdl functionality in reverse cholesterol transport--challenges in translating data emerging from mouse models to human disease. Biochim Biophys Acta. 2016;1861:566–583 - PubMed
-
- Kosmas CE, Christodoulidis G, Cheng JW, Vittorio TJ, Lerakis S. High-density lipoprotein functionality in coronary artery disease. Am J Med Sci. 2014;347:504–508 - PubMed
-
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein ai. Nature. 1991;353:265–267 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

