Turbulent Flow Promotes Cleavage of VWF (von Willebrand Factor) by ADAMTS13 (A Disintegrin and Metalloproteinase With a Thrombospondin Type-1 Motif, Member 13)
- PMID: 31291760
- PMCID: PMC9109938
- DOI: 10.1161/ATVBAHA.119.312814
Turbulent Flow Promotes Cleavage of VWF (von Willebrand Factor) by ADAMTS13 (A Disintegrin and Metalloproteinase With a Thrombospondin Type-1 Motif, Member 13)
Abstract
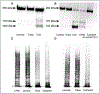
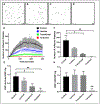
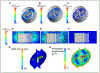
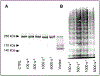
Objective- Acquired von Willebrand syndrome is defined by excessive cleavage of the VWF (von Willebrand Factor) and is associated with impaired primary hemostasis and severe bleeding. It often develops when blood is exposed to nonphysiological flow such as in aortic stenosis or mechanical circulatory support. We evaluated the role of laminar, transitional, and turbulent flow on VWF cleavage and the effects on VWF function. Approach and Results- We used a vane rheometer to generate laminar, transitional, and turbulent flow and evaluate the effect of each on VWF cleavage in the presence of ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type-1 motif, member 13). We performed functional assays to evaluate the effect of these flows on VWF structure and function. Computational fluid dynamics was used to estimate the flow fields and forces within the vane rheometer under each flow condition. Turbulent flow is required for excessive cleavage of VWF in an ADAMTS13-dependent manner. The assay was repeated with whole blood, and the turbulent flow had the same effect. Our computational fluid dynamics results show that under turbulent conditions, the Kolmogorov scale approaches the size of VWF. Finally, cleavage of VWF in this study has functional consequences under flow as the resulting VWF has decreased ability to bind platelets and collagen. Conclusions- Turbulent flow mediates VWF cleavage in the presence of ADAMTS13, decreasing the ability of VWF to sustain platelet adhesion. These findings impact the design of mechanical circulatory support devices and are relevant to pathological environments where turbulence is added to circulation.
Keywords: aortic valve stenosis; collagen; hemostasis; von Willebrand Factor.
Figures

Comment in
-
Gone With the Vane.Arterioscler Thromb Vasc Biol. 2019 Sep;39(9):1702-1704. doi: 10.1161/ATVBAHA.119.313110. Epub 2019 Aug 21. Arterioscler Thromb Vasc Biol. 2019. PMID: 31433699 Free PMC article. No abstract available.
References
-
- Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. - PubMed
-
- Peterson DM, Stathopoulos NA, Giorgio TD, Hellums JD, Moake JL. Shear-induced platelet aggregation requires von willebrand factor and platelet membrane glycoproteins Ib and IIb-IIIa. Blood. 1987;69:625–628. - PubMed
-
- Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von willebrand factor. Blood. 1996;88:2939–2950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

