Neuroinflammation: friend and foe for ischemic stroke
- PMID: 31291966
- PMCID: PMC6617684
- DOI: 10.1186/s12974-019-1516-2
Neuroinflammation: friend and foe for ischemic stroke
Abstract
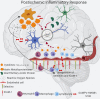
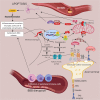
Stroke, the third leading cause of death and disability worldwide, is undergoing a change in perspective with the emergence of new ideas on neurodegeneration. The concept that stroke is a disorder solely of blood vessels has been expanded to include the effects of a detrimental interaction between glia, neurons, vascular cells, and matrix components, which is collectively referred to as the neurovascular unit. Following the acute stroke, the majority of which are ischemic, there is secondary neuroinflammation that both promotes further injury, resulting in cell death, but conversely plays a beneficial role, by promoting recovery. The proinflammatory signals from immune mediators rapidly activate resident cells and influence infiltration of a wide range of inflammatory cells (neutrophils, monocytes/macrophages, different subtypes of T cells, and other inflammatory cells) into the ischemic region exacerbating brain damage. In this review, we discuss how neuroinflammation has both beneficial as well as detrimental roles and recent therapeutic strategies to combat pathological responses. Here, we also focus on time-dependent entry of immune cells to the ischemic area and the impact of other pathological mediators, including oxidative stress, excitotoxicity, matrix metalloproteinases (MMPs), high-mobility group box 1 (HMGB1), arachidonic acid metabolites, mitogen-activated protein kinase (MAPK), and post-translational modifications that could potentially perpetuate ischemic brain damage after the acute injury. Understanding the time-dependent role of inflammatory factors could help in developing new diagnostic, prognostic, and therapeutic neuroprotective strategies for post-stroke inflammation.
Keywords: Blood-brain barrier; Ischemia; Neuroinflammation; Stroke.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Rammal SA, Almekhlafi MA. Diabetes mellitus and stroke in the Arab world. J Taibah University Med Sci. 2016;11(4):295–300. doi: 10.1016/j.jtumed.2016.05.001. - DOI
-
- Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877–897. doi: 10.1016/S1474-4422(17)30299-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

