Characterisation of a niche-specific excretory-secretory peroxiredoxin from the parasitic nematode Teladorsagia circumcincta
- PMID: 31292008
- PMCID: PMC6617597
- DOI: 10.1186/s13071-019-3593-6
Characterisation of a niche-specific excretory-secretory peroxiredoxin from the parasitic nematode Teladorsagia circumcincta
Abstract
Background: The primary cause of parasitic gastroenteritis in small ruminants in temperate regions is the brown stomach worm, Teladorsagia circumcincta. Host immunity to this parasite is slow to develop, consistent with the ability of T. circumcincta to suppress the host immune response. Previous studies have shown that infective fourth-stage T. circumcincta larvae produce excretory-secretory products that are able to modulate the host immune response. The objective of this study was to identify immune modulatory excretory-secretory proteins from populations of fourth-stage T. circumcincta larvae present in two different host-niches: those associated with the gastric glands (mucosal-dwelling larvae) and those either loosely associated with the mucosa or free-living in the lumen (lumen-dwelling larvae).
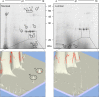
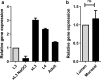
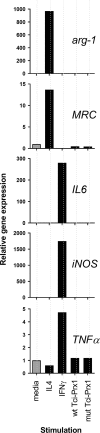
Results: In this study excretory-secretory proteins from mucosal-dwelling and lumen-dwelling T. circumcincta fourth stage larvae were analysed using comparative 2-dimensional gel electrophoresis. A total of 17 proteins were identified as differentially expressed, with 14 proteins unique to, or enriched in, the excretory-secretory proteins of mucosal-dwelling larvae. One of the identified proteins, unique to mucosal-dwelling larvae, was a putative peroxiredoxin (T. circumcincta peroxiredoxin 1, Tci-Prx1). Peroxiredoxin orthologs from the trematode parasites Schistosoma mansoni and Fasciola hepatica have previously been shown to alternatively activate macrophages and play a key role in promoting parasite induced Th2 type immunity. Here we demonstrate that Tci-Prx1 is expressed in all infective T. circumcincta life-stages and, when produced as a recombinant protein, has peroxidase activity, whereby hydrogen peroxide (H2O2) is reduced and detoxified. Furthermore, we use an in vitro macrophage stimulation assay to demonstrate that, unlike peroxiredoxins from trematode parasites Schistosoma mansoni and Fasciola hepatica, Tci-Prx1 is unable to alternatively activate murine macrophage cells.
Conclusions: In this study, we identified differences in the excretory-secretory proteome of mucosal-dwelling and lumen-dwelling infective fourth-stage T. circumcincta larvae, and demonstrated the utility of this comparative proteomic approach to identify excretory-secretory proteins of potential importance for parasite survival and/or host immune modulation.
Keywords: Anti-oxidant; Excretory–secretory; Niche; Peroxidase; Peroxiredoxin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Sommerville RI. The histotropic phase of the nematode parasite, Ostertagia circumcincta. Aust J Agric Res. 1954;5:130–140. doi: 10.1071/AR9540130. - DOI
-
- McNeilly TN, Frew D, Burgess STG, Wright H, Bartley DJ, Bartley Y, et al. Niche-specific gene expression in a parasitic nematode; increased expression of immunomodulators in Teladorsagia circumcincta larvae derived from host mucosa. Sci Rep. 2017;7:7214. doi: 10.1038/s41598-017-07092-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

