Safety and feasibility of virus-specific T cells derived from umbilical cord blood in cord blood transplant recipients
- PMID: 31292125
- PMCID: PMC6650740
- DOI: 10.1182/bloodadvances.2019000201
Safety and feasibility of virus-specific T cells derived from umbilical cord blood in cord blood transplant recipients
Erratum in
-
Abraham AA, John TD, Keller MD, et al. Safety and feasibility of virus-specific T cells derived from umbilical cord blood in cord blood transplant recipients. Blood Adv. 2019;3(14):2057-2068.Blood Adv. 2019 Aug 27;3(16):2453. doi: 10.1182/bloodadvances.2019000741. Blood Adv. 2019. PMID: 31420368 Free PMC article. No abstract available.
Abstract
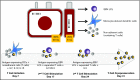
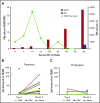
Adoptive transfer of virus-specific T cells (VSTs) has been shown to be safe and effective in stem cell transplant recipients. However, the lack of virus-experienced T cells in donor cord blood (CB) has prevented the development of ex vivo expanded donor-derived VSTs for recipients of this stem cell source. Here we evaluated the feasibility and safety of ex vivo expansion of CB T cells from the 20% fraction of the CB unit in pediatric patients receiving a single CB transplant (CBT). In 2 clinical trials conducted at 2 separate sites, we manufactured CB-derived multivirus-specific T cells (CB-VSTs) targeting Epstein-Barr virus (EBV), adenovirus, and cytomegalovirus (CMV) for 18 (86%) of 21 patients demonstrating feasibility. Manufacturing for 2 CB-VSTs failed to meet lot release because of insufficient cell recovery, and there was 1 sterility breach during separation of the frozen 20% fraction. Delayed engraftment was not observed in patients who received the remaining 80% fraction for the primary CBT. There was no grade 3 to 4 acute graft-versus-host disease (GVHD) associated with the infusion of CB-VSTs. None of the 7 patients who received CB-VSTs as prophylaxis developed end-organ disease from CMV, EBV, or adenovirus. In 7 patients receiving CB-VSTs for viral reactivation or infection, only 1 patient developed end-organ viral disease, which was in an immune privileged site (CMV retinitis) and occurred after steroid therapy for GVHD. Finally, we demonstrated the long-term persistence of adoptively transferred CB-VSTs using T-cell receptor-Vβ clonotype tracking, suggesting that CB-VSTs are a feasible addition to antiviral pharmacotherapy.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.M.B. is on the scientific advisory board (SAB) for Cellectis, has stock options in Neximmune and Torque Therapeutics, and is a cofounder and SAB member of Mana Therapeutics. P.J.H., C.R.N.C., M.D.K., and C.M.B. have patents related to this work. P.J.H. and C.R.N.C. are cofounders of Mana Therapeutics and serve on the Board (P.J.H.) or the SAB (C.R.N.C.). The remaining authors declare no competing financial interests.
Figures



References
-
- Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238-241. - PubMed
-
- Rooney CM, Smith CA, Ng CY, et al. . Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9-13. - PubMed
-
- Leen AM, Myers GD, Sili U, et al. . Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160-1166. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

