DNA-SWCNT Biosensors Allow Real-Time Monitoring of Therapeutic Responses in Pancreatic Ductal Adenocarcinoma
- PMID: 31292162
- PMCID: PMC6726513
- DOI: 10.1158/0008-5472.CAN-18-3337
DNA-SWCNT Biosensors Allow Real-Time Monitoring of Therapeutic Responses in Pancreatic Ductal Adenocarcinoma
Abstract
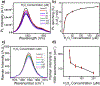
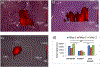
Pancreatic ductal adenocarcinoma (PDAC) is a highly desmoplastic cancer with limited treatment options. There is an urgent need for tools that monitor therapeutic responses in real time. Drugs such as gemcitabine and irinotecan elicit their therapeutic effect in cancer cells by producing hydrogen peroxide (H2O2). In this study, specific DNA-wrapped single-walled carbon nanotubes (SWCNT), which precisely monitor H2O2, were used to determine the therapeutic response of PDAC cells in vitro and tumors in vivo. Drug therapeutic efficacy was evaluated in vitro by monitoring H2O2 differences in situ using reversible alteration of Raman G-bands from the nanotubes. Implantation of the DNA-SWCNT probe inside the PDAC tumor resulted in approximately 50% reduction of Raman G-band intensity when treated with gemcitabine versus the pretreated tumor; the Raman G-band intensity reversed to its pretreatment level upon treatment withdrawal. In summary, using highly specific and sensitive DNA-SWCNT nanosensors, which can determine dynamic alteration of hydrogen peroxide in tumor, can evaluate the effectiveness of chemotherapeutics. SIGNIFICANCE: A novel biosensor is used to detect intratumoral hydrogen peroxide, allowing real-time monitoring of responses to chemotherapeutic drugs.
©2019 American Association for Cancer Research.
Conflict of interest statement
Figures


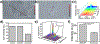

Similar articles
-
Simvastatin attenuates macrophage-mediated gemcitabine resistance of pancreatic ductal adenocarcinoma by regulating the TGF-β1/Gfi-1 axis.Cancer Lett. 2017 Jan 28;385:65-74. doi: 10.1016/j.canlet.2016.11.006. Epub 2016 Nov 11. Cancer Lett. 2017. PMID: 27840243
-
Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma.Cancer Lett. 2017 Sep 10;403:296-304. doi: 10.1016/j.canlet.2017.06.026. Epub 2017 Jul 4. Cancer Lett. 2017. PMID: 28687352 Free PMC article.
-
Enhancing sorafenib-mediated sensitization to gemcitabine in experimental pancreatic cancer through EMAP II.J Exp Clin Cancer Res. 2013 Mar 6;32(1):12. doi: 10.1186/1756-9966-32-12. J Exp Clin Cancer Res. 2013. PMID: 23497499 Free PMC article.
-
Inhibiting signal transducer and activator of transcription-3 increases response to gemcitabine and delays progression of pancreatic cancer.Mol Cancer. 2013 Sep 11;12(1):104. doi: 10.1186/1476-4598-12-104. Mol Cancer. 2013. PMID: 24025152 Free PMC article.
-
Current Standard and Future Perspectives in First- and Second-Line Treatment of Metastatic Pancreatic Adenocarcinoma.Digestion. 2016;94(1):44-9. doi: 10.1159/000447739. Epub 2016 Jul 21. Digestion. 2016. PMID: 27438590 Review.
Cited by
-
Biosensing with Fluorescent Carbon Nanotubes.Angew Chem Int Ed Engl. 2022 Apr 25;61(18):e202112372. doi: 10.1002/anie.202112372. Epub 2022 Mar 1. Angew Chem Int Ed Engl. 2022. PMID: 34978752 Free PMC article. Review.
-
Enhancing Optical Properties and Stability of DNA-Functionalized Carbon Nanotubes with Cryoprotectant-Mediated Lyophilization.bioRxiv [Preprint]. 2025 Aug 31:2025.08.26.672410. doi: 10.1101/2025.08.26.672410. bioRxiv. 2025. PMID: 40909615 Free PMC article. Preprint.
-
Near-Infrared Fluorescent Single-Walled Carbon Nanotubes for Biosensing.Small. 2025 Jul;21(26):e2502542. doi: 10.1002/smll.202502542. Epub 2025 May 2. Small. 2025. PMID: 40317978 Free PMC article. Review.
References
-
- Lee SH, Kim OK, Lee S, Kim JK. Local-dependency of morphological and optical properties between breast cancer cell lines. Spectrochim Acta A Mol Biomol Spectrosc 2018;205:132–8 - PubMed
-
- Whatcott CJ, Posner RG, Von Hoff DD, Han H. Desmoplasia and chemoresistance in pancreatic cancer. In: Grippo PJ, Munshi HG, editors. Pancreatic Cancer and Tumor Microenvironment; Trivandrum (India)2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

