Dosage reduction and discontinuation of biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis: protocol for a pragmatic, randomised controlled trial (the BIOlogical Dose OPTimisation (BIODOPT) trial)
- PMID: 31292181
- PMCID: PMC6624054
- DOI: 10.1136/bmjopen-2018-028517
Dosage reduction and discontinuation of biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis: protocol for a pragmatic, randomised controlled trial (the BIOlogical Dose OPTimisation (BIODOPT) trial)
Abstract
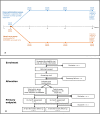
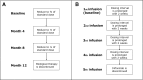
Introduction: The The BIOlogical Dose OPTimisation (BIODOPT) trial is a pragmatic, multicentre, randomised controlled, open-label, parallel-group, equivalence study designed to evaluate tapering of biological disease-modifying antirheumatic drugs (bDMARDs) in patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) in sustained clinical remission or low disease activity (LDA). Traditionally, these patients maintain standard dosage of bDMARD lifelong; however, recent studies indicate that a significant proportion of patients in sustained remission or LDA can taper their bDMARD and maintain stable disease activity. Thus, this trial aims to evaluate whether a disease activity-guided tapering strategy for bDMARDs will enable a significant dosage reduction while maintaining disease activity compared with usual care. From the individual patient's standpoint as well as from a societal perspective, it would be advantageous if bDMARDs could be reduced or even discontinued while maintaining disease activity.
Methods and analysis: A total of 180 patients with RA, PsA or axSpA treated with bDMARDs and in clinical remission/LDA during the past 12 months will be enrolled from four centres in Denmark. Patients will be randomised in a ratio of 2:1 to either disease activity-guided tapering of bDMARDs (intervention group) or continuation of bDMARDs as usual care (control group).The primary objective is the difference between the two groups in the proportion of patients who have reduced their inclusion dosage of bDMARDs to 50% or less while maintaining stable disease activity at 18 months follow-up.
Ethics and dissemination: The study is approved by the ethics committee of Northern Jutland, Denmark (N-20170073) and by the Danish Medicine Agency. Patient research partner KHH contributed to refinement of the protocol and approved the final manuscript. Results will be disseminated through publication in international peer-reviewed journals.
Trial registration number: 2017-001970-41; Pre-results.
Keywords: axial spondyloarthritis; biological therapy; dosage reduction; psoriatic arthritis; rheumatoid arthritis; tapering.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LU has received speaker honoraria from Abbvie, Eli Lilly and Novartis (not related to the submitted work). SK has received speaker honoraria from Novartis and Eli Lilly (not related to the submitted work). AS has received speaker honoraria from MSD and Eli Lilly (not related to the submitted work). LD has received speaker honoraria from UCB, MSD, Eli Lilly and Janssen Pharmaceutica (not related to the submitted work).
Figures
Similar articles
-
Exploring TNFi drug-levels and anti-drug antibodies during tapering among patients with inflammatory arthritis: secondary analyses from the randomised BIODOPT trial.Rheumatol Int. 2024 Oct;44(10):1897-1908. doi: 10.1007/s00296-024-05665-7. Epub 2024 Jul 24. Rheumatol Int. 2024. PMID: 39043980 Free PMC article. Clinical Trial.
-
High disease relapse after bDMARD spacing in psoriatic arthritis compared to rheumatoid arthritis and axial spondyloarthritis patients: real-life data from BIOPURE registry.Clin Rheumatol. 2021 Sep;40(9):3659-3665. doi: 10.1007/s10067-021-05728-1. Epub 2021 Apr 16. Clin Rheumatol. 2021. PMID: 33864158
-
Design of a phase IV randomised, double-blind, placebo-controlled trial assessing the ImPact of Residual Inflammation Detected via Imaging TEchniques, Drug Levels and Patient Characteristics on the Outcome of Dose TaperIng of Adalimumab in Clinical Remission Rheumatoid ArThritis (RA) patients (PREDICTRA).BMJ Open. 2018 Feb 28;8(2):e019007. doi: 10.1136/bmjopen-2017-019007. BMJ Open. 2018. PMID: 29490959 Free PMC article. Clinical Trial.
-
Risk of losing remission, low disease activity or radiographic progression in case of bDMARD discontinuation or tapering in rheumatoid arthritis: systematic analysis of the literature and meta-analysis.Ann Rheum Dis. 2018 Apr;77(4):515-522. doi: 10.1136/annrheumdis-2017-212423. Epub 2017 Nov 29. Ann Rheum Dis. 2018. PMID: 29187350
-
Impact of tapering targeted therapies (bDMARDs or JAKis) on the risk of serious infections and adverse events of special interest in patients with rheumatoid arthritis or spondyloarthritis: a systematic analysis of the literature and meta-analysis.Arthritis Res Ther. 2020 Apr 29;22(1):97. doi: 10.1186/s13075-020-02188-x. Arthritis Res Ther. 2020. PMID: 32349791 Free PMC article.
Cited by
-
Understanding flare in axial spondyloarthritis: novel insights from daily self-reported flare experience.Rheumatol Adv Pract. 2021 Nov 15;5(3):rkab082. doi: 10.1093/rap/rkab082. eCollection 2021. Rheumatol Adv Pract. 2021. PMID: 34926981 Free PMC article.
-
Tumour necrosis factor inhibitor dose adaptation in psoriatic arthritis and axial spondyloarthritis (TAPAS): a retrospective cohort study.Rheumatology (Oxford). 2022 May 30;61(6):2307-2315. doi: 10.1093/rheumatology/keab741. Rheumatology (Oxford). 2022. PMID: 34599803 Free PMC article.
-
Exploring TNFi drug-levels and anti-drug antibodies during tapering among patients with inflammatory arthritis: secondary analyses from the randomised BIODOPT trial.Rheumatol Int. 2024 Oct;44(10):1897-1908. doi: 10.1007/s00296-024-05665-7. Epub 2024 Jul 24. Rheumatol Int. 2024. PMID: 39043980 Free PMC article. Clinical Trial.
-
Appropriate Injection Intervals of Dupilumab in Patients With Adult Atopic Dermatitis: A Step Toward Developing Guidelines for Daily Practice.Ann Dermatol. 2025 Feb;37(1):39-45. doi: 10.5021/ad.24.084. Ann Dermatol. 2025. PMID: 39894672 Free PMC article.
-
Disease Activity-Guided Stepwise Tapering but Not Discontinuation of Biologics Is a Feasible Therapeutic Strategy for Patients with Ankylosing Spondylitis: Real-World Evidence.Adv Ther. 2022 Mar;39(3):1393-1402. doi: 10.1007/s12325-021-01995-1. Epub 2022 Feb 2. Adv Ther. 2022. PMID: 35106691
References
-
- Singh J, Hossain A, Tanjong Ghogomu E, et al. . Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease modifying anti-rheumatic drugs: a systematic review and network meta-analysis. Cochrane Database Syst Rev 2016;5:CD012183. - PMC - PubMed
-
- Mease PJ, Fleischmann R, Deodhar AA, et al. . Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. 10.1136/annrheumdis-2013-203696 - DOI - PMC - PubMed
-
- Dougados M, van der Heijde D, Sieper J, et al. . Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2014;66:2091–102. 10.1002/art.38721 - DOI - PubMed
-
- Landewé R, Braun J, Deodhar A, et al. . Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014;73:39–47. 10.1136/annrheumdis-2013-204231 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
