Comparative quantitative systems pharmacology modeling of anti-PCSK9 therapeutic modalities in hypercholesterolemia
- PMID: 31292220
- PMCID: PMC6718444
- DOI: 10.1194/jlr.M092486
Comparative quantitative systems pharmacology modeling of anti-PCSK9 therapeutic modalities in hypercholesterolemia
Abstract
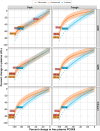
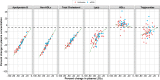
Since the discovery of proprotein convertase subtilisin/kexin type 9 (PCSK9) as an attractive target in the treatment of hypercholesterolemia, multiple anti-PCSK9 therapeutic modalities have been pursued in drug development. The objective of this research is to set the stage for the quantitative benchmarking of two anti-PCSK9 pharmacological modality classes, monoclonal antibodies (mAbs) and small interfering RNA (siRNA). To this end, we developed an integrative mathematical model of lipoprotein homeostasis describing the dynamic interplay between PCSK9, LDL-cholesterol (LDL-C), VLDL-cholesterol, HDL-cholesterol (HDL-C), apoB, lipoprotein a [Lp(a)], and triglycerides (TGs). We demonstrate that LDL-C decreased proportionally to PCSK9 reduction for both mAb and siRNA modalities. At marketed doses, however, treatment with mAbs resulted in an additional ∼20% LDL-C reduction compared with siRNA. We further used the model as an evaluation tool and determined that no quantitative differences were observed in HDL-C, Lp(a), TG, or apoB responses, suggesting that the disruption of PCSK9 synthesis would provide no additional effects on lipoprotein-related biomarkers in the patient segment investigated. Predictive model simulations further indicate that siRNA therapies may reach reductions in LDL-C levels comparable to those achieved with mAbs if the current threshold of 80% PCSK9 inhibition via siRNA could be overcome.
Keywords: atherosclerosis; cholesterol metabolism; lipoproteins; plasma proprotein convertase subtilisin/kexin type 9; quantitative modeling; translational pharmacology.
Copyright © 2019 Sokolov et al.
Figures


References
-
- Camejo G., Hurt-Camejo E., Wiklund O., and Bondjers G.. 1998. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 139: 205–222. - PubMed
-
- Stroes E. S., Thompson P. D., Corsini A., Vladutiu G. D., Raal F. J., Ray K. K., Roden M., Stein E., Tokgözoğlu L., Nordestgaard B. G., et al. 2015. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 36: 1012–1022. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

