Suppression of the photoparoxysmal response in photosensitive epilepsy with cenobamate (YKP3089)
- PMID: 31292226
- PMCID: PMC6709996
- DOI: 10.1212/WNL.0000000000007894
Suppression of the photoparoxysmal response in photosensitive epilepsy with cenobamate (YKP3089)
Abstract
Objective: To evaluate the effect of cenobamate in patients with photoparoxysmal-EEG response (PPR) to intermittent photic stimulation (IPS) as proof of principle of efficacy in patients with epilepsy.
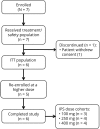
Methods: In this multicenter, single-blind study, adults with photosensitive epilepsy, with/without concomitant antiepileptic drug therapy, underwent IPS under 3 eye conditions after a single dose of placebo (day -1, day 2) or cenobamate (day 1; 100, 250, or 400 mg). Complete suppression was a standardized photosensitivity range reduction to 0 over ≥1 time points for all eye conditions. Partial suppression was a ≥3-point reduction over ≥3 testing times vs the same time points on day -1 in ≥1 eye condition. Pharmacokinetics and safety were assessed.
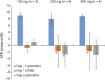
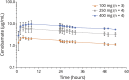
Results: Of 6 evaluable patients, 5 reentered to receive higher doses. Cenobamate 100 mg produced partial suppression in 1 of 3 patients; 250 mg produced complete suppression in 1 of 4 and partial suppression in 4 of 4 patients; and 400 mg produced complete suppression in 1 of 4 and partial suppression in 2 of 4 patients. PPR was consistently reduced on days 1 and 2 (>24 hours after cenobamate) vs day -1 (placebo) with the 250- and 400-mg doses. Area under the plasma concentration-time curve (before dose to last measurable concentration) values between 201 and 400 μg/h/mL resulted in partial suppression in 4 of 6 (66%) patients. Most common adverse events were dizziness and somnolence.
Conclusions: This proof-of-principle study demonstrated that cenobamate is a potentially effective product for epilepsy.
Clinicaltrialsgov identifier: NCT00616148.
Classification of evidence: This study provides Class III evidence that, for patients with photosensitive epilepsy, cenobamate suppresses IPS-induced PPR.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Binnie CD, Kasteleijn-Nolst Trenite DG, De Korte R. Photosensitivity as a model for acute antiepileptic drug studies. Electroencephalogr Clin Neurophysiol 1986;63:35–41. - PubMed
-
- Di Prospero NA, Gambale JJ, Pandina G, et al. Evaluation of JNJ-26489112 in patients with photosensitive epilepsy: a placebo-controlled, exploratory study. Epilepsy Res 2014;108:709–716. - PubMed
-
- Kasteleijn-Nolst Trenite D, Genton P, Brandt C, Reed RC. The “photosensitivity model” is (also) a model for focal (partial) seizures. Epilepsy Res 2017;133:113–120. - PubMed
-
- Lu Y, Waltz S, Stenzel K, Muhle H, Stephani U. Photosensitivity in epileptic syndromes of childhood and adolescence. Epileptic Disord 2008;10:136–143. - PubMed
-
- Kasteleijn-Nolst Trenite DG, van Emde Boas W, Groenhout CM, Meinardi H. Preliminary assessment of the efficacy of Org 6370 in photosensitive epileptic patients: paradoxical enhancement of photosensitivity and provocation of myoclonic seizures. Epilepsia 1992;33:135–141. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
