Crimean-Congo Hemorrhagic Fever Mouse Model Recapitulating Human Convalescence
- PMID: 31292241
- PMCID: PMC6714788
- DOI: 10.1128/JVI.00554-19
Crimean-Congo Hemorrhagic Fever Mouse Model Recapitulating Human Convalescence
Abstract
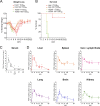
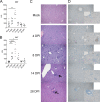
Crimean-Congo hemorrhagic fever virus (CCHFV) is a cause of severe hemorrhagic fever. Its tick reservoir and vector are widely distributed throughout Africa, Southern and Eastern Europe, the Middle East, and Asia. Serological evidence suggests that CCHFV can productively infect a wide variety of species, but only humans develop severe, sometimes fatal disease. The role of the host adaptive immunity in control or contribution to the severe pathology seen in CCHF cases is largely unknown. Studies of adaptive immune responses to CCHFV have been limited due to lack of suitable small animal models. Wild-type mice are resistant to CCHFV infection, and type I interferon-deficient mice typically develop a rapid-onset fatal disease prior to development of adaptive immune responses. We report here a mouse model in which type I interferon-deficient mice infected with a clinical isolate of CCHFV develop a severe inflammatory disease but ultimately recover. Recovery was coincident with development of CCHFV-specific B- and T-cell responses that were sustained for weeks postinfection. We also found that recovery from a primary CCHFV infection could protect against disease following homologous or heterologous reinfection. Together this model enables study of multiple aspects of CCHFV pathogenesis, including convalescence, an important aspect of CCHF disease that existing mouse models have been unsuitable for studying.IMPORTANCE The role of antibody or virus-specific T-cell responses in control of acute Crimean-Congo hemorrhagic fever virus infection is largely unclear. This is a critical gap in our understanding of CCHF, and investigation of convalescence following severe acute CCHF has been limited by the lack of suitable small animal models. We report here a mouse model of CCHF in which infected mice develop severe disease but ultimately recover. Although mice developed an inflammatory immune response along with severe liver and spleen pathology, these mice also developed CCHFV-specific B- and T-cell responses and were protected from reinfection. This model provides a valuable tool to investigate how host immune responses control acute CCHFV infection and how these responses may contribute to the severe disease seen in CCHFV-infected humans in order to develop therapeutic interventions that promote protective immune responses.
Keywords: Crimean-Congo hemorrhagic fever virus; adaptive immunity; animal models; inflammation; pathogenesis.
Copyright © 2019 American Society for Microbiology.
Figures

Similar articles
-
Immunocompetent mouse model for Crimean-Congo hemorrhagic fever virus.Elife. 2021 Jan 8;10:e63906. doi: 10.7554/eLife.63906. Elife. 2021. PMID: 33416494 Free PMC article.
-
Nucleoside-Modified mRNA Vaccines Protect IFNAR-/- Mice against Crimean-Congo Hemorrhagic Fever Virus Infection.J Virol. 2022 Feb 9;96(3):e0156821. doi: 10.1128/JVI.01568-21. Epub 2021 Nov 24. J Virol. 2022. PMID: 34817199 Free PMC article.
-
Immunization with DNA Plasmids Coding for Crimean-Congo Hemorrhagic Fever Virus Capsid and Envelope Proteins and/or Virus-Like Particles Induces Protection and Survival in Challenged Mice.J Virol. 2017 Apr 28;91(10):e02076-16. doi: 10.1128/JVI.02076-16. Print 2017 May 15. J Virol. 2017. PMID: 28250124 Free PMC article.
-
Recent advances in understanding Crimean-Congo hemorrhagic fever virus.F1000Res. 2018 Oct 29;7:F1000 Faculty Rev-1715. doi: 10.12688/f1000research.16189.1. eCollection 2018. F1000Res. 2018. PMID: 30416710 Free PMC article. Review.
-
Animal Models for Crimean-Congo Hemorrhagic Fever Human Disease.Viruses. 2019 Jun 28;11(7):590. doi: 10.3390/v11070590. Viruses. 2019. PMID: 31261754 Free PMC article. Review.
Cited by
-
Vaccination with the Crimean-Congo hemorrhagic fever virus viral replicon vaccine induces NP-based T-cell activation and antibodies possessing Fc-mediated effector functions.Front Cell Infect Microbiol. 2023 Aug 21;13:1233148. doi: 10.3389/fcimb.2023.1233148. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37671145 Free PMC article.
-
Pathogenicity of Lloviu and Bombali Viruses in Type I Interferon Receptor Knockout Mice.J Infect Dis. 2023 Nov 13;228(Suppl 7):S548-S553. doi: 10.1093/infdis/jiad226. J Infect Dis. 2023. PMID: 37352146 Free PMC article.
-
Crimean-Congo Hemorrhagic Fever Virus (CCHFV): A Silent but Widespread Threat.Curr Trop Med Rep. 2021;8(2):141-147. doi: 10.1007/s40475-021-00235-4. Epub 2021 Mar 16. Curr Trop Med Rep. 2021. PMID: 33747715 Free PMC article. Review.
-
Immunobiology of Crimean-Congo hemorrhagic fever.Antiviral Res. 2022 Mar;199:105244. doi: 10.1016/j.antiviral.2022.105244. Epub 2022 Jan 11. Antiviral Res. 2022. PMID: 35026307 Free PMC article. Review.
-
Host response transcriptomic analysis of Crimean-Congo hemorrhagic fever pathogenesis in the cynomolgus macaque model.Sci Rep. 2021 Oct 6;11(1):19807. doi: 10.1038/s41598-021-99130-1. Sci Rep. 2021. PMID: 34615921 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

