Replicons of a Rodent Hepatitis C Model Virus Permit Selection of Highly Permissive Cells
- PMID: 31292246
- PMCID: PMC6744251
- DOI: 10.1128/JVI.00733-19
Replicons of a Rodent Hepatitis C Model Virus Permit Selection of Highly Permissive Cells
Abstract
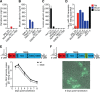
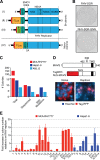
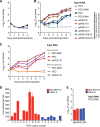
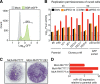
Animal hepaciviruses represent promising surrogate models for hepatitis C virus (HCV), for which there are no efficient immunocompetent animal models. Experimental infection of laboratory rats with rodent hepacivirus isolated from feral Rattus norvegicus (RHV-rn1) mirrors key aspects of HCV infection in humans, including chronicity, hepatitis, and steatosis. Moreover, RHV has been adapted to infect immunocompetent laboratory mice. RHV in vitro systems have not been developed but would enable detailed studies of the virus life cycle crucial for designing animal experiments to model HCV infection. Here, we established efficient RHV-rn1 selectable subgenomic replicons with and without reporter genes. Rat and mouse liver-derived cells did not readily support the complete RHV life cycle, but replicon-containing cell clones could be selected with and without acquired mutations. Replication was significantly enhanced by mutations in NS4B and NS5A and in cell clones cured of replicon RNA. These mutations increased RHV replication of both mono- and bicistronic constructs, and CpG/UpA-dinucleotide optimization of reporter genes allowed replication. Using the replicon system, we show that the RHV-rn1 NS3-4A protease cleaves a human mitochondrial antiviral signaling protein reporter, providing a sensitive readout for virus replication. RHV-rn1 replication was inhibited by the HCV polymerase inhibitor sofosbuvir and high concentrations of HCV NS5A antivirals but not by NS3 protease inhibitors. The microRNA-122 antagonist miravirsen inhibited RHV-rn1 replication, demonstrating the importance of this HCV host factor for RHV. These novel RHV in vitro systems will be useful for studies of tropism, molecular virology, and characterization of virus-host interactions, thereby providing important complements to in vivo systems.IMPORTANCE A vaccine against hepatitis C virus (HCV) is crucial for global control of this important pathogen, which induces fatal human liver diseases. Vaccine development has been hampered by the lack of immunocompetent animal models. Discovery of rodent hepacivirus (RHV) enabled establishment of novel surrogate animal models. These allow robust infection and reverse genetic and immunization studies of laboratory animals, which develop HCV-like chronicity. Currently, there are no RHV in vitro systems available to study tropism and molecular virology. Here, we established the first culture systems for RHV, recapitulating the intracellular phase of the virus life cycle in vitro These replicon systems enabled identification of replication-enhancing mutations and selection of cells highly permissive to RHV replication, which allow study of virus-host interactions. HCV antivirals targeting NS5A, NS5B, and microRNA-122 efficiently inhibited RHV replication. Hence, several important aspects of HCV replication are shared by the rodent virus system, reinforcing its utility as an HCV model.
Keywords: animal models; antiviral agents; hepacivirus; hepatitis C virus; micro-RNA; replication; replicon.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Viral persistence, liver disease, and host response in a hepatitis C-like virus rat model.Hepatology. 2018 Aug;68(2):435-448. doi: 10.1002/hep.29494. Epub 2018 May 21. Hepatology. 2018. PMID: 28859226 Free PMC article.
-
Priming of Antiviral CD8 T Cells without Effector Function by a Persistently Replicating Hepatitis C-Like Virus.J Virol. 2020 May 4;94(10):e00035-20. doi: 10.1128/JVI.00035-20. Print 2020 May 4. J Virol. 2020. PMID: 32102885 Free PMC article.
-
Neutralization and receptor use of infectious culture-derived rat hepacivirus as a model for HCV.Hepatology. 2022 Nov;76(5):1506-1519. doi: 10.1002/hep.32535. Epub 2022 May 12. Hepatology. 2022. PMID: 35445423 Free PMC article.
-
The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control.J Hepatol. 2016 Oct;65(1 Suppl):S2-S21. doi: 10.1016/j.jhep.2016.07.035. J Hepatol. 2016. PMID: 27641985 Review.
-
Hepatitis C virus resistance to protease inhibitors.J Hepatol. 2011 Jul;55(1):192-206. doi: 10.1016/j.jhep.2011.01.011. Epub 2011 Feb 1. J Hepatol. 2011. PMID: 21284949 Review.
Cited by
-
Effect of direct-acting antivirals on the titers of human pegivirus 1 during treatment of chronic hepatitis C patients.Microbiol Spectr. 2024 Sep 3;12(9):e0064124. doi: 10.1128/spectrum.00641-24. Epub 2024 Jul 25. Microbiol Spectr. 2024. PMID: 39051781 Free PMC article.
-
Design and nonviral delivery of live attenuated vaccine to prevent chronic hepatitis C virus-like infection.Nat Commun. 2025 Aug 15;16(1):7629. doi: 10.1038/s41467-025-62813-8. Nat Commun. 2025. PMID: 40817105 Free PMC article.
-
Adenovirus-vectored T cell vaccine for hepacivirus shows reduced effectiveness against a CD8 T cell escape variant in rats.PLoS Pathog. 2021 Mar 18;17(3):e1009391. doi: 10.1371/journal.ppat.1009391. eCollection 2021 Mar. PLoS Pathog. 2021. PMID: 33735321 Free PMC article.
-
Versatile SARS-CoV-2 Reverse-Genetics Systems for the Study of Antiviral Resistance and Replication.Viruses. 2022 Jan 18;14(2):172. doi: 10.3390/v14020172. Viruses. 2022. PMID: 35215765 Free PMC article.
-
Animal Models Used in Hepatitis C Virus Research.Int J Mol Sci. 2020 May 29;21(11):3869. doi: 10.3390/ijms21113869. Int J Mol Sci. 2020. PMID: 32485887 Free PMC article. Review.
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–1858. doi:10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
-
- Bartenschlager R, Baumert TF, Bukh J, Houghton M, Lemon SM, Lindenbach BD, Lohmann V, Moradpour D, Pietschmann T, Rice CM, Thimme R, Wakita T. 2018. Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: considerations for scientists and funding agencies. Virus Res 248:53–62. doi:10.1016/j.virusres.2018.02.016. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

