The Leader Protein of Theiler's Virus Prevents the Activation of PKR
- PMID: 31292248
- PMCID: PMC6744238
- DOI: 10.1128/JVI.01010-19
The Leader Protein of Theiler's Virus Prevents the Activation of PKR
Abstract
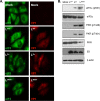
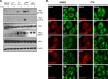
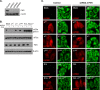
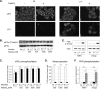
Leader (L) proteins encoded by cardioviruses are multifunctional proteins that contribute to innate immunity evasion. L proteins of Theiler's murine encephalomyelitis virus (TMEV), Saffold virus (SAFV), and encephalomyocarditis virus (EMCV) were reported to inhibit stress granule assembly in infected cells. Here, we show that TMEV L can act at two levels in the stress granule formation pathway: on the one hand, it can inhibit sodium arsenite-induced stress granule assembly without preventing eIF2α phosphorylation and, thus, acts downstream of eIF2α; on the other hand, it can inhibit eucaryotic translation initiation factor 2 alpha kinase 2 (PKR) activation and the consequent PKR-mediated eIF2α phosphorylation. Interestingly, coimmunostaining experiments revealed that PKR colocalizes with viral double-stranded RNA (dsRNA) in cells infected with L-mutant viruses but not in cells infected with the wild-type virus. Furthermore, PKR coprecipitated with dsRNA from cells infected with L-mutant viruses significantly more than from cells infected with the wild-type virus. These data strongly suggest that L blocks PKR activation by preventing the interaction between PKR and viral dsRNA. In infected cells, L also rendered PKR refractory to subsequent activation by poly(I·C). However, no interaction was observed between L and either dsRNA or PKR. Taken together, our results suggest that, unlike other viral proteins, L indirectly acts on PKR to negatively regulate its responsiveness to dsRNA.IMPORTANCE The leader (L) protein encoded by cardioviruses is a very short multifunctional protein that contributes to evasion of the host innate immune response. This protein notably prevents the formation of stress granules in infected cells. Using Theiler's virus as a model, we show that L proteins can act at two levels in the stress response pathway leading to stress granule formation, the most striking one being the inhibition of eucaryotic translation initiation factor 2 alpha kinase 2 (PKR) activation. Interestingly, the leader protein appears to inhibit PKR via a novel mechanism by rendering this kinase unable to detect double-stranded RNA, its typical activator. Unlike other viral proteins, such as influenza virus NS1, the leader protein appears to interact with neither PKR nor double-stranded RNA, suggesting that it acts indirectly to trigger the inhibition of the kinase.
Keywords: PKR; Theiler's murine encephalomyelitis virus; cardiovirus; double-stranded RNA virus; leader protein; picornavirus.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
The leader protein of cardioviruses inhibits stress granule assembly.J Virol. 2011 Sep;85(18):9614-22. doi: 10.1128/JVI.00480-11. Epub 2011 Jul 13. J Virol. 2011. PMID: 21752908 Free PMC article.
-
Regulation of PACT-Mediated Protein Kinase Activation by the OV20.0 Protein of Orf Virus.J Virol. 2015 Nov;89(22):11619-29. doi: 10.1128/JVI.01739-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355092 Free PMC article.
-
Opposing Roles of Double-Stranded RNA Effector Pathways and Viral Defense Proteins Revealed with CRISPR-Cas9 Knockout Cell Lines and Vaccinia Virus Mutants.J Virol. 2016 Aug 12;90(17):7864-79. doi: 10.1128/JVI.00869-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334583 Free PMC article.
-
Viral proteins targeting host protein kinase R to evade an innate immune response: a mini review.Biotechnol Genet Eng Rev. 2018 Apr;34(1):33-59. doi: 10.1080/02648725.2018.1467151. Epub 2018 May 2. Biotechnol Genet Eng Rev. 2018. PMID: 29716441 Review.
-
Activation of PKR: an open and shut case?Trends Biochem Sci. 2007 Feb;32(2):57-62. doi: 10.1016/j.tibs.2006.12.003. Epub 2006 Dec 29. Trends Biochem Sci. 2007. PMID: 17196820 Free PMC article. Review.
Cited by
-
Coronavirus Endoribonuclease Ensures Efficient Viral Replication and Prevents Protein Kinase R Activation.J Virol. 2021 Apr 1;95(7):e02103-20. doi: 10.1128/JVI.02103-20. Epub 2020 Dec 23. J Virol. 2021. PMID: 33361429 Free PMC article.
-
A case of convergent evolution: Several viral and bacterial pathogens hijack RSK kinases through a common linear motif.Proc Natl Acad Sci U S A. 2022 Feb 1;119(5):e2114647119. doi: 10.1073/pnas.2114647119. Proc Natl Acad Sci U S A. 2022. PMID: 35091472 Free PMC article.
-
Seneca Valley Virus 3C Protease Inhibits Stress Granule Formation by Disrupting eIF4GI-G3BP1 Interaction.Front Immunol. 2020 Sep 29;11:577838. doi: 10.3389/fimmu.2020.577838. eCollection 2020. Front Immunol. 2020. PMID: 33133097 Free PMC article.
-
Controlling reactogenicity while preserving immunogenicity from a self-amplifying RNA vaccine by modulating nucleocytoplasmic transport.NPJ Vaccines. 2025 Apr 29;10(1):85. doi: 10.1038/s41541-025-01135-8. NPJ Vaccines. 2025. PMID: 40301369 Free PMC article.
-
SARS-CoV-2 nucleocapsid protein inhibits the PKR-mediated integrated stress response through RNA-binding domain N2b.PLoS Pathog. 2023 Aug 22;19(8):e1011582. doi: 10.1371/journal.ppat.1011582. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37607209 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

