LAP+ Cells Modulate Protection Induced by Oral Vaccination with Rhesus Rotavirus in a Neonatal Mouse Model
- PMID: 31292251
- PMCID: PMC6744246
- DOI: 10.1128/JVI.00882-19
LAP+ Cells Modulate Protection Induced by Oral Vaccination with Rhesus Rotavirus in a Neonatal Mouse Model
Abstract
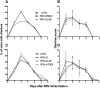
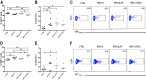
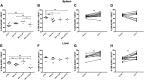
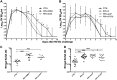
Transforming growth factor β (TGF-β) has been shown to play a role in immunity against different pathogens in vitro and against parasites in vivo However, its role in viral infections in vivo is incompletely understood. Using a neonatal mouse model of heterologous rhesus rotavirus (RV) vaccination, we show that the vaccine induced rotavirus-specific CD4 T cells, the majority of which lacked expression of KLRG1 or CD127, and a few regulatory rotavirus-specific CD4 T cells that expressed surface latency-associated peptide (LAP)-TGF-β. In these mice, inhibiting TGF-β, with both a neutralizing antibody and an inhibitor of TGF-β receptor signaling (activin receptor-like kinase 5 inhibitor [ALK5i]), did not change the development or intensity of the mild diarrhea induced by the vaccine, the rotavirus-specific T cell response, or protection against a subsequent challenge with a murine EC-rotavirus. However, mice treated with anti-LAP antibodies had improved protection after a homologous EC-rotavirus challenge, compared with control rhesus rotavirus-immunized mice. Thus, oral vaccination with a heterologous rotavirus stimulates regulatory RV-specific CD4 LAP-positive (LAP+) T cells, and depletion of LAP+ cells increases vaccine-induced protection.IMPORTANCE Despite the introduction of several live attenuated animal and human rotaviruses as efficient oral vaccines, rotaviruses continue to be the leading etiological agent for diarrhea mortality among children under 5 years of age worldwide. Improvement of these vaccines has been partially delayed because immunity to rotaviruses is incompletely understood. In the intestine (where rotavirus replicates), regulatory T cells that express latency-associated peptide (LAP) play a prominent role, which has been explored for many diseases but not specifically for infectious agents. In this paper, we show that neonatal mice given a live oral rotavirus vaccine develop rotavirus-specific LAP+ T cells and that depletion of these cells improves the efficiency of the vaccine. These findings may prove useful for the design of strategies to improve rotavirus vaccines.
Keywords: intestine; latency-associated peptide; neonatal mice; rotavirus; transforming growth factor β; vaccine.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
Inclusion of a universal tetanus toxoid CD4(+) T cell epitope P2 significantly enhanced the immunogenicity of recombinant rotavirus ΔVP8* subunit parenteral vaccines.Vaccine. 2014 Jul 31;32(35):4420-4427. doi: 10.1016/j.vaccine.2014.06.060. Epub 2014 Jun 21. Vaccine. 2014. PMID: 24962749 Free PMC article.
-
Possible mechanisms of protection elicited by candidate rotavirus vaccines as determined with the adult mouse model.Viral Immunol. 2003;16(1):17-24. doi: 10.1089/088282403763635410. Viral Immunol. 2003. PMID: 12725685 Review.
-
CD4+CD25- T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism.J Immunol. 2003 Mar 1;170(5):2516-22. doi: 10.4049/jimmunol.170.5.2516. J Immunol. 2003. PMID: 12594277
-
Mice develop effective but delayed protective immune responses when immunized as neonates either intranasally with nonliving VP6/LT(R192G) or orally with live rhesus rotavirus vaccine candidates.J Virol. 2006 May;80(10):4949-61. doi: 10.1128/JVI.80.10.4949-4961.2006. J Virol. 2006. PMID: 16641286 Free PMC article.
-
Prospects for development of a rotavirus vaccine against rotavirus diarrhea in infants and young children.Rev Infect Dis. 1989 May-Jun;11 Suppl 3:S539-46. doi: 10.1093/clinids/11.supplement_3.s539. Rev Infect Dis. 1989. PMID: 2548276 Review.
Cited by
-
Isolation and identification of BRV G6P[1] strain in Heilongjiang province, Northeast China.Front Vet Sci. 2024 Sep 20;11:1416465. doi: 10.3389/fvets.2024.1416465. eCollection 2024. Front Vet Sci. 2024. PMID: 39372897 Free PMC article.
-
Update on Early-Life T Cells: Impact on Oral Rotavirus Vaccines.Viruses. 2024 May 22;16(6):818. doi: 10.3390/v16060818. Viruses. 2024. PMID: 38932111 Free PMC article. Review.
References
-
- Franco MA. 2016. Papel del TGF-β en la inmunidad contra los rotavirus. Rev Acad Colomb Cienc Ex Fis Nat 40:18–26. doi:10.18257/raccefyn.300. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

