Translational impact of NIH-funded nonhuman primate research in transplantation
- PMID: 31292263
- PMCID: PMC7197021
- DOI: 10.1126/scitranslmed.aau0143
Translational impact of NIH-funded nonhuman primate research in transplantation
Abstract
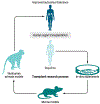
The National Institutes of Health (NIH) has long supported using nonhuman primate (NHP) models for research on kidney, pancreatic islet, heart, and lung transplantation. The primary purpose of this research has been to develop new treatments for down-modulating or preventing deleterious immune responses after transplantation in human patients. Here, we discuss NIH-funded NHP studies of immune cell depletion, costimulation blockade, regulatory cell therapy, desensitization, and mixed hematopoietic chimerism that either preceded clinical trials or prevented the human application of therapies that were toxic or ineffective.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
References
-
- Cosimi AB, Clinical application of tolerance induction in solid organ transplantation. Transplant. Proc 31, 1803–1805 (1999). - PubMed
-
- Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW Jr., Colvin RB, Sykes M, Sachs DH, HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med 358, 353–361 (2008). - PMC - PubMed
-
- Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC, Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 381,434–438 (1996). - PubMed
-
- Preston EH, Xu H, Dhanireddy KK, Pearl JP, Leopardi FV, Starost MF, Hale DA, Kirk AD, IDEC-131 (anti-CD154), sirolimus and donor-specific transfusion facilitate operational tolerance in non-human primates. Am. J. Transplant 5, 1032–1041 (2005). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

