Changes in plasma lipids predict pravastatin efficacy in secondary prevention
- PMID: 31292301
- PMCID: PMC6629250
- DOI: 10.1172/jci.insight.128438
Changes in plasma lipids predict pravastatin efficacy in secondary prevention
Abstract
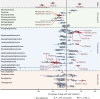
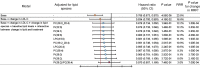
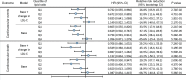
BACKGROUNDStatins have pleiotropic effects on lipid metabolism. The relationship between these effects and future cardiovascular events is unknown. We characterized the changes in lipids upon pravastatin treatment and defined the relationship with risk reduction for future cardiovascular events.METHODSPlasma lipids (n = 342) were measured in baseline and 1-year follow-up samples from a Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) study subcohort (n = 4991). The associations of changes in lipids with treatment and cardiovascular outcomes were investigated using linear and Cox regression. The effect of treatment on future cardiovascular outcomes was examined by the relative risk reduction (RRR).RESULTSPravastatin treatment was associated with changes in 206 lipids. Species containing arachidonic acid were positively associated while phosphatidylinositol species were negatively associated with pravastatin treatment. The RRR from pravastatin treatment for cardiovascular events decreased from 23.5% to 16.6% after adjustment for clinical risk factors and change in LDL-cholesterol (LDL-C) and to 3.0% after further adjustment for the change in the lipid ratio PI(36:2)/PC(38:4). Change in PI(36:2)/PC(38:4) mediated 58% of the treatment effect. Stratification of patients into quartiles of change in PI(36:2)/PC(38:4) indicated no benefit of pravastatin in the fourth quartile.CONCLUSIONThe change in PI(36:2)/PC(38:4) predicted benefit from pravastatin, independent of change in LDL-C, demonstrating its potential as a biomarker for monitoring the clinical benefit of statin treatment in secondary prevention.TRIAL REGISTRATIONAustralian New Zealand Clinical Trials Registry identifier ACTRN12616000535471.FUNDINGBristol-Myers Squibb; NHMRC grants 211086, 358395, and 1029754; NHMRC program grant 1149987; NHMRC fellowship 108026; and the Operational Infrastructure Support Program of the Victorian government of Australia.
Keywords: Cardiovascular disease; Cholesterol; Clinical Trials; Clinical practice; Metabolism.
Conflict of interest statement
Figures

References
-
- Scott R. Lipid modifying agents: mechanisms of action and reduction of cardiovascular disease. Clin Exp Pharmacol Physiol. 1997;24(5):A26–A28. - PubMed
-
- Cholesterol Treatment Trialists’ (CTT) Collaborators, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5. - DOI - PMC - PubMed
-
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

