m6A enhances the phase separation potential of mRNA
- PMID: 31292544
- PMCID: PMC6662915
- DOI: 10.1038/s41586-019-1374-1
m6A enhances the phase separation potential of mRNA
Abstract
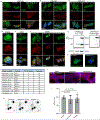
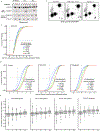
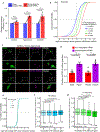
N6-methyladenosine (m6A) is the most prevalent modified nucleotide in mRNA1,2, with around 25% of mRNAs containing at least one m6A. Methylation of mRNA to form m6A is required for diverse cellular and physiological processes3. Although the presence of m6A in an mRNA can affect its fate in different ways, it is unclear how m6A directs this process and why the effects of m6A can vary in different cellular contexts. Here we show that the cytosolic m6A-binding proteins-YTHDF1, YTHDF2 and YTHDF3-undergo liquid-liquid phase separation in vitro and in cells. This phase separation is markedly enhanced by mRNAs that contain multiple, but not single, m6A residues. Polymethylated mRNAs act as a multivalent scaffold for the binding of YTHDF proteins, juxtaposing their low-complexity domains and thereby leading to phase separation. The resulting mRNA-YTHDF complexes then partition into different endogenous phase-separated compartments, such as P-bodies, stress granules or neuronal RNA granules. m6A-mRNA is subject to compartment-specific regulation, including a reduction in the stability and translation of mRNA. These studies reveal that the number and distribution of m6A sites in cellular mRNAs can regulate and influence the composition of the phase-separated transcriptome, and suggest that the cellular properties of m6A-modified mRNAs are governed by liquid-liquid phase separation principles.
Conflict of interest statement
Figures








References
-
- Perry RP & Kelley DE Existence of methylated messenger RNA in mouse L cells. Cell 1, 37–42 (1974).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

