Evaluating Screening Participation, Follow-up, and Outcomes for Breast, Cervical, and Colorectal Cancer in the PROSPR Consortium
- PMID: 31292633
- PMCID: PMC7073922
- DOI: 10.1093/jnci/djz137
Evaluating Screening Participation, Follow-up, and Outcomes for Breast, Cervical, and Colorectal Cancer in the PROSPR Consortium
Abstract
Background: Cancer screening is a complex process encompassing risk assessment, the initial screening examination, diagnostic evaluation, and treatment of cancer precursors or early cancers. Metrics that enable comparisons across different screening targets are needed. We present population-based screening metrics for breast, cervical, and colorectal cancers for nine sites participating in the Population-based Research Optimizing Screening through Personalized Regimens consortium.
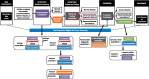
Methods: We describe how selected metrics map to a trans-organ conceptual model of the screening process. For each cancer type, we calculated calendar year 2013 metrics for the screen-eligible target population (breast: ages 40-74 years; cervical: ages 21-64 years; colorectal: ages 50-75 years). Metrics for screening participation, timely diagnostic evaluation, and diagnosed cancers in the screened and total populations are presented for the total eligible population and stratified by age group and cancer type.
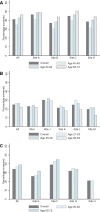
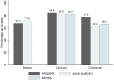
Results: The overall screening-eligible populations in 2013 were 305 568 participants for breast, 3 160 128 for cervical, and 2 363 922 for colorectal cancer screening. Being up-to-date for testing was common for all three cancer types: breast (63.5%), cervical (84.6%), and colorectal (77.5%). The percentage of abnormal screens ranged from 10.7% for breast, 4.4% for cervical, and 4.5% for colorectal cancer screening. Abnormal breast screens were followed up diagnostically in almost all (96.8%) cases, and cervical and colorectal were similar (76.2% and 76.3%, respectively). Cancer rates per 1000 screens were 5.66, 0.17, and 1.46 for breast, cervical, and colorectal cancer, respectively.
Conclusions: Comprehensive assessment of metrics by the Population-based Research Optimizing Screening through Personalized Regimens consortium enabled systematic identification of screening process steps in need of improvement. We encourage widespread use of common metrics to allow interventions to be tested across cancer types and health-care settings.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
The Future Is Prosperous.J Natl Cancer Inst. 2020 Mar 1;112(3):219-220. doi: 10.1093/jnci/djz139. J Natl Cancer Inst. 2020. PMID: 31292643 Free PMC article. No abstract available.
References
-
- Bulliard JL, Garcia M, Blom J, Senore C, Mai V, Klabunde C.. Sorting out measures and definitions of screening participation to improve comparability: the example of colorectal cancer. Eur J Cancer. 2014;50(2):434–446. - PubMed
-
- Klabunde C, Blom J, Bulliard JL, et al. Participation rates for organized colorectal cancer screening programmes: an international comparison. J Med Screen. 2015;22(3):119–126. - PubMed
-
- Cancer Care Ontario. Ontario Cancer Screening Performance Report 2016. Toronto: Cancer Care Ontario; 2016.

