Biomarkers and subtypes of deranged lipid metabolism in non-alcoholic fatty liver disease
- PMID: 31293337
- PMCID: PMC6603806
- DOI: 10.3748/wjg.v25.i24.3009
Biomarkers and subtypes of deranged lipid metabolism in non-alcoholic fatty liver disease
Abstract
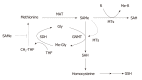
Nonalcoholic fatty liver disease (NAFLD) is a heterogeneous and complex disease that is imprecisely diagnosed by liver biopsy. NAFLD covers a spectrum that ranges from simple steatosis, nonalcoholic steatohepatitis (NASH) with varying degrees of fibrosis, to cirrhosis, which is a major risk factor for hepatocellular carcinoma. Lifestyle and eating habit changes during the last century have made NAFLD the most common liver disease linked to obesity, type 2 diabetes mellitus and dyslipidemia, with a global prevalence of 25%. NAFLD arises when the uptake of fatty acids (FA) and triglycerides (TG) from circulation and de novo lipogenesis saturate the rate of FA β-oxidation and very-low density lipoprotein (VLDL)-TG export. Deranged lipid metabolism is also associated with NAFLD progression from steatosis to NASH, and therefore, alterations in liver and serum lipidomic signatures are good indicators of the disease's development and progression. This review focuses on the importance of the classification of NAFLD patients into different subtypes, corresponding to the main alteration(s) in the major pathways that regulate FA homeostasis leading, in each case, to the initiation and progression of NASH. This concept also supports the targeted intervention as a key approach to maximize therapeutic efficacy and opens the door to the development of precise NASH treatments.
Keywords: Lipid metabolism; Lipidomics; Methionine adenosyltransferase; Multiomics; Nonalcoholic steatohepatitis; One-carbon metabolism; Precision medicine; S-adenosylmethionine; Steatosis; Very low-density lipoproteins.
Conflict of interest statement
Conflict-of-interest statement: The authors have declared no conflicts of interest.
Figures