Development and in vitro study of a bi-specific magnetic resonance imaging molecular probe for hepatocellular carcinoma
- PMID: 31293339
- PMCID: PMC6603812
- DOI: 10.3748/wjg.v25.i24.3030
Development and in vitro study of a bi-specific magnetic resonance imaging molecular probe for hepatocellular carcinoma
Abstract
Background: Hepatocellular carcinoma (HCC) ranks second in terms of cancer mortality worldwide. Molecular magnetic resonance imaging (MRI) targeting HCC biomarkers such as alpha-fetoprotein (AFP) or glypican-3 (GPC3) offers new strategies to enhance specificity and help early diagnosis of HCC. However, the existing iron oxide nanoparticle-based MR molecular probes singly target AFP or GPC3, which may hinder their efficiency to detect heterogeneous micro malignant HCC tumors < 1 cm (MHCC). We hypothesized that the strategy of double antibody-conjugated iron oxide nanoparticles which simultaneously target AFP and GPC3 antigens may potentially be used to overcome the tumor heterogeneity and enhance the detection rate for MRI-based MHCC diagnosis.
Aim: To synthesize an AFP/GPC3 double antibody-labeled iron oxide MRI molecular probe and to assess its impact on MRI specificity and sensitivity at the cellular level.
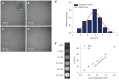
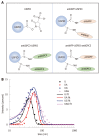
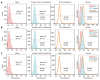
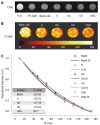
Methods: A double antigen-targeted MRI probe for MHCC anti-AFP-USPIO-anti-GPC3 (UAG) was developed by simultaneously conjugating AFP andGPC3 antibodies to a 5 nm ultra-small superparamagnetic iron oxide nanoparticle (USPIO). At the same time, the singly labeled probes of anti-AFP-USPIO (UA) and anti-GPC3-USPIO (UG) and non-targeted USPIO (U) were also prepared for comparison. The physical characterization including morphology (transmission electron microscopy), hydrodynamic size, and zeta potential (dynamic light scattering) was conducted for each of the probes. The antigen targeting and MRI ability for these four kinds of USPIO probes were studied in the GPC3-expressing murine hepatoma cell line Hepa1-6/GPC3. First, AFP and GPC3 antigen expression in Hepa1-6/GPC3 cells was confirmed by flow cytometry and immunocytochemistry. Then, the cellular uptake of USPIO probes was investigated by Prussian blue staining assay and in vitro MRI (T2-weighted and T2-map) with a 3.0 Tesla clinical MR scanner.
Results: Our data showed that the double antibody-conjugated probe UAG had the best specificity in targeting Hepa1-6/GPC3 cells expressing AFP and GPC3 antigens compared with single antibody-conjugated and unconjugated USPIO probes. The iron Prussian blue staining and quantitative T2-map MRI analysis showed that, compared with UA, UG, and U, the uptake of double antigen-targeted UAG probe demonstrated a 23.3% (vs UA), 15.4% (vs UG), and 57.3% (vs U) increased Prussian stained cell percentage and a 14.93% (vs UA), 9.38% (vs UG), and 15.3% (vs U) reduction of T2 relaxation time, respectively. Such bi-specific probe might have the potential to overcome tumor heterogeneity. Meanwhile, the coupling of two antibodies did not influence the magnetic performance of USPIO, and the relatively small hydrodynamic size (59.60 ± 1.87 nm) of double antibody-conjugated USPIO probe makes it a viable candidate for use in MHCC MRI in vivo, as they are slowly phagocytosed by macrophages.
Conclusion: The bi-specific probe presents enhanced targeting efficiency and MRI sensitivity to HCC cells than singly- or non-targeted USPIO, paving the way for in vivo translation to further evaluate its clinical potential.
Keywords: Alpha-fetoprotein; Glypican-3; Hepatocellular carcinoma; Magnetic resonance imaging; Molecular imaging; Ultra-small superparamagnetic iron nanoparticles.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that there are no conflicts of interest regarding the publication of the paper.
Figures



Similar articles
-
Bi-specific T1 positive-contrast-enhanced magnetic resonance imaging molecular probe for hepatocellular carcinoma in an orthotopic mouse model.World J Gastrointest Oncol. 2022 Apr 15;14(4):858-871. doi: 10.4251/wjgo.v14.i4.858. World J Gastrointest Oncol. 2022. PMID: 35582105 Free PMC article.
-
Preparation of magnetic resonance probes using one-pot method for detection of hepatocellular carcinoma.World J Gastroenterol. 2015 Apr 14;21(14):4275-83. doi: 10.3748/wjg.v21.i14.4275. World J Gastroenterol. 2015. PMID: 25892879 Free PMC article.
-
A GPC3-specific aptamer-mediated magnetic resonance probe for hepatocellular carcinoma.Int J Nanomedicine. 2018 Aug 1;13:4433-4443. doi: 10.2147/IJN.S168268. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30122918 Free PMC article.
-
Ultrasmall superparamagnetic iron oxide-anti-CD20 monoclonal antibody.2007 May 21 [updated 2008 Feb 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 May 21 [updated 2008 Feb 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641470 Free Books & Documents. Review.
-
Usefulness of the novel oncofetal antigen glypican-3 for diagnosis of hepatocellular carcinoma and melanoma.BioDrugs. 2005;19(2):71-7. doi: 10.2165/00063030-200519020-00001. BioDrugs. 2005. PMID: 15807627 Review.
Cited by
-
Advances in the early diagnosis of hepatocellular carcinoma.Genes Dis. 2020 Jan 27;7(3):308-319. doi: 10.1016/j.gendis.2020.01.014. eCollection 2020 Sep. Genes Dis. 2020. PMID: 32884985 Free PMC article. Review.
-
Targeted Molecular Imaging Probes Based on Magnetic Resonance Imaging for Hepatocellular Carcinoma Diagnosis and Treatment.Biosensors (Basel). 2022 May 17;12(5):342. doi: 10.3390/bios12050342. Biosensors (Basel). 2022. PMID: 35624643 Free PMC article. Review.
-
Nanotechnology-Assisted Cell Tracking.Nanomaterials (Basel). 2022 Apr 20;12(9):1414. doi: 10.3390/nano12091414. Nanomaterials (Basel). 2022. PMID: 35564123 Free PMC article. Review.
-
Preparation of a Novel Multifunctional Cationic Liposome Drug-carrying System and its Functional Study on Lung Cancer.Anticancer Agents Med Chem. 2024;24(14):1085-1095. doi: 10.2174/0118715206294695240522075454. Anticancer Agents Med Chem. 2024. PMID: 38803174
-
Preoperative prediction of glypican-3 positive expression in solitary hepatocellular carcinoma on gadoxetate-disodium enhanced magnetic resonance imaging.Front Immunol. 2022 Aug 25;13:973153. doi: 10.3389/fimmu.2022.973153. eCollection 2022. Front Immunol. 2022. PMID: 36091074 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. - PubMed
-
- Laurent S, Henoumont C, Stanicki D, Boutry S, Lipani E, Belaid S, Muller RN, Elst LV. MRI Applications: Classification According to Their Biodistribution. SpringerBriefs in Applied Sciences and Technology. In: MRI Contrast Agents., editor. Singapore: Springer; 2017. pp. 111–125.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

