Semiparametric Estimation of Task-Based Dynamic Functional Connectivity on the Population Level
- PMID: 31293367
- PMCID: PMC6598619
- DOI: 10.3389/fnins.2019.00583
Semiparametric Estimation of Task-Based Dynamic Functional Connectivity on the Population Level
Abstract
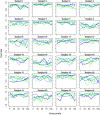
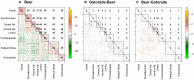
Dynamic functional connectivity (dFC) estimates time-dependent associations between pairs of brain region time series as typically acquired during functional MRI. dFC changes are most commonly quantified by pairwise correlation coefficients between the time series within a sliding window. Here, we applied a recently developed bootstrap-based technique (Kudela et al., 2017) to robustly estimate subject-level dFC and its confidence intervals in a task-based fMRI study (24 subjects who tasted their most frequently consumed beer and Gatorade as an appetitive control). We then combined information across subjects and scans utilizing semiparametric mixed models to obtain a group-level dFC estimate for each pair of brain regions, flavor, and the difference between flavors. The proposed approach relies on the estimated group-level dFC accounting for complex correlation structures of the fMRI data, multiple repeated observations per subject, experimental design, and subject-specific variability. It also provides condition-specific dFC and confidence intervals for the whole brain at the group level. As a summary dFC metric, we used the proportion of time when the estimated associations were either significantly positive or negative. For both flavors, our fully-data driven approach yielded regional associations that reflected known, biologically meaningful brain organization as shown in prior work, as well as closely resembled resting state networks (RSNs). Specifically, beer flavor-potentiated associations were detected between several reward-related regions, including the right ventral striatum (VST), lateral orbitofrontal cortex, and ventral anterior insular cortex (vAIC). The enhancement of right VST-vAIC association by a taste of beer independently validated the main activation-based finding (Oberlin et al., 2016). Most notably, our novel dFC methodology uncovered numerous associations undetected by the traditional static FC analysis. The data-driven, novel dFC methodology presented here can be used for a wide range of task-based fMRI designs to estimate the dFC at multiple levels-group-, individual-, and task-specific, utilizing a combination of well-established statistical methods.
Keywords: addiction; dynamic functional connectivity; functional MRI; gustatory task; semiparametric mixed models; statistical methods.
Figures





References
-
- Adali T., Anderson M., Fu G. S. (2014). Diversity in independent component and vector analyses: identifiability, algorithms, and applications in medical imaging. IEEE Signal Process. Mag. 31, 18–33. 10.1109/MSP.2014.2300511 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

