Silencing of SNHG12 Enhanced the Effectiveness of MSCs in Alleviating Ischemia/Reperfusion Injuries via the PI3K/AKT/mTOR Signaling Pathway
- PMID: 31293373
- PMCID: PMC6603177
- DOI: 10.3389/fnins.2019.00645
Silencing of SNHG12 Enhanced the Effectiveness of MSCs in Alleviating Ischemia/Reperfusion Injuries via the PI3K/AKT/mTOR Signaling Pathway
Abstract
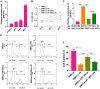
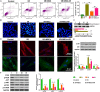
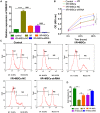
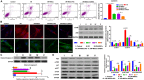
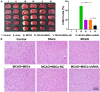
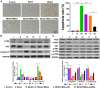
Previous studies have reported that the long non-coding RNA SNHG12 (lncRNA SNHG12) plays a critical role in regulating the function of mesenchymal stem cells (MSCs); however, the effect of lncRNA SNHG12 on MSCs in injured brain tissue has rarely been reported. We studied the effect and mechanism of lncRNA SNHG12-modified mesenchymal stem cells (MSCs) in treating brain injuries caused by ischemia/reperfusion (I/R). I/R treated rat brain microvascular endothelial cells (BMECs) were co-cultured with MSCs or I/R pretreated MSCs. Next, BMEC proliferation was detected by using CCK-8 and EdU assays, and cell apoptosis was determined by using flow cytometry and the Hoechst staining method. Autophagy of BMECs was determined using immunofluorescence and expression of associated pathway proteins were measured by western blotting. Moreover, BMEC proliferation, apoptosis, and autophagy were also determined after the BMECs had been co-cultured with shSNHG12-MSCs. In addition, a rat model of middle cerebral artery occlusion (MCAO) was used to further confirm the findings obtained with cells. I/R treatment significantly decreased the proliferation of BMECs, but increased their levels of SNHG12 expression, apoptosis, and autophagy. However, co-culturing of BMECs with MSCs markedly alleviated the reduction in BMEC proliferation and the increases in BMEC apoptosis and autophagy, as well as the phosphorylation of PI3K, AKT, and mTOR proteins in BMECs that had been induced by I/R. Furthermore, shSNHG12 remarkably enhanced the effects of MSCs. In addition, an injection MSCs reduced the infarct areas and rates of cell apoptosis in MACO rats, and reduced the phosphorylation of PI3K, AKT, and mTOR proteins. Moreover, shSNHG12 enhanced the ameliorative effect of MSCs in treating brain injuries in the MACO rats. In conclusion, silencing of SNHG12 enhanced the effects of MSCs in reducing apoptosis and autophagy of BMECs by activating the PI3K/AKT/mTOR signaling pathway.
Keywords: SNHG12; apoptosis; autophagy; ischemia/reperfusion injury; mesenchymal stem cell.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

