Tau Secretion: Good and Bad for Neurons
- PMID: 31293374
- PMCID: PMC6606725
- DOI: 10.3389/fnins.2019.00649
Tau Secretion: Good and Bad for Neurons
Abstract
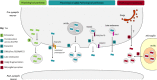
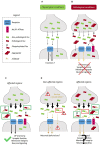
In Alzheimer's disease (AD), neurofibrillary tangles (NFTs), lesions composed of hyperphosphorylated and aggregated tau, spread from the transentorhinal cortex to the hippocampal formation and neocortex. Growing evidence indicates that tau pathology propagates trans-synaptically, implying that pathological tau released by pre-synaptic neurons is taken up by post-synaptic neurons where it accumulates and aggregates. Observations such as the presence of tau in the cerebrospinal fluid (CSF) from control individuals and in the CSF of transgenic mice overexpressing human tau before the detection of neuronal death indicate that tau can be secreted by neurons. The increase of tau in the CSF in pathological conditions such as AD suggests that tau secretion is enhanced and/or other secretory pathways take place when neuronal function is compromised. In physiological conditions, extracellular tau could exert beneficial effects as observed for other cytosolic proteins also released in the extracellular space. In such a case, blocking tau secretion could have negative effects on neurons unless the mechanism of tau secretion are different in physiological and pathological conditions allowing the prevention of pathological tau secretion without affecting the secretion of physiological tau. Furthermore, distinct extracellular tau species could be secreted in physiological and pathological conditions, species having the capacity to induce tau pathology being only secreted in the latter condition. In the present review, we will focus on the mechanisms and function of tau secretion in both physiological and pathological conditions and how this information can help to elaborate an efficient therapeutic strategy to prevent tau pathology and its propagation.
Keywords: extracellular tau; propagation; secretion; tau protein; tauopathies.
Figures
References
-
- Barthelemy N. R., Gabelle A., Hirtz C., Fenaille F., Sergeant N., Schraen-Maschke S., et al. (2016). Differential mass spectrometry profiles of tau protein in the cerebrospinal fluid of patients with Alzheimer’s disease, progressive supranuclear palsy, and dementia with lewy bodies. J. Alzheimers Dis. 51 1033–1043. 10.3233/jad-150962 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

