Alzheimer Disease Pathogenesis: Insights From Molecular and Cellular Biology Studies of Oligomeric Aβ and Tau Species
- PMID: 31293377
- PMCID: PMC6598402
- DOI: 10.3389/fnins.2019.00659
Alzheimer Disease Pathogenesis: Insights From Molecular and Cellular Biology Studies of Oligomeric Aβ and Tau Species
Abstract
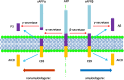
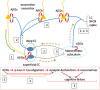
Alzheimer disease (AD) represents an oncoming epidemic that without an effective treatment promises to exact extraordinary human and financial burdens. Studies of pathogenesis are essential for defining targets for discovering disease-modifying treatments. Past studies of AD neuropathology provided valuable, albeit limited, insights. Nevertheless, building on these findings, recent studies have provided an increasingly rich harvest of genetic, molecular and cellular data that are creating unprecedented opportunities to both understand and treat AD. Among the most significant are those documenting the presence within the AD brain of toxic oligomeric species of Aβ and tau. Existing data support the view that such species can propagate and spread within neural circuits. To place these findings in context we first review the genetics and neuropathology of AD, including AD in Down syndrome (AD-DS). We detail studies that support the existence of toxic oligomeric species while noting the significant unanswered questions concerning their precise structures, the means by which they spread and undergo amplification and how they induce neuronal dysfunction and degeneration. We conclude by offering a speculative synthesis for how oligomers of Aβ and tau initiate and drive pathogenesis. While 100 years after Alzheimer's first report there is much still to learn about pathogenesis and the discovery of disease-modifying treatments, the application of new concepts and sophisticated new tools are poised to deliver important advances for combatting AD.
Keywords: Alzheimer disease; Aβ; Down syndrome; PET; amyloid plaques; neurofibrillary tangles; oligomer; tau.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

