Quercetin Enhances Inhibitory Synaptic Inputs and Reduces Excitatory Synaptic Inputs to OFF- and ON-Type Retinal Ganglion Cells in a Chronic Glaucoma Rat Model
- PMID: 31293381
- PMCID: PMC6604910
- DOI: 10.3389/fnins.2019.00672
Quercetin Enhances Inhibitory Synaptic Inputs and Reduces Excitatory Synaptic Inputs to OFF- and ON-Type Retinal Ganglion Cells in a Chronic Glaucoma Rat Model
Abstract
Background: Glaucoma is a neurodegenerative disease caused by excitotoxic injury of retinal ganglion cells (RGCs). In our previous model of high intraocular pressure, prepared by injecting magnetic beads into the anterior chamber, we demonstrated that an important natural dietary flavonoid compound (quercetin) can improve RGC function. However, it is unclear whether quercetin can improve the synaptic function of RGCs and how quercetin regulates synaptic transmission in rat models of chronic glaucoma.
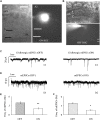
Methods: A rat model of chronic glaucoma was prepared by electrocoagulation of the superior scleral vein. Electrophysiological electroretinography was used to detect the photopic negative response (PhNR). The whole-cell patch-clamp technique was used to clamp ON- and OFF- type RGCs in sections from normal retinas and from retinas that had been subjected to glaucoma for 4 weeks.
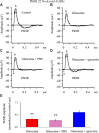
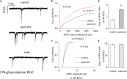
Results: Quercetin can reverse the decrease in PhNR amplitude caused by chronic glaucoma. The baseline frequency of miniature GABAergic inhibitory postsynaptic currents (mIPSCs) in the RGCs of glaucomatous retinal slices was lower than that of the control group. The frequencies of miniature excitatory postsynaptic currents (mEPSCs) were not significantly different between control and glaucomatous RGCs. The baseline frequencies of GABAergic mIPSCs and mEPSCs in OFF-type glaucomatous RGCs were greater than those in ON-type glaucomatous RGCs. Quercetin increased miniature GABAergic inhibitory neurotransmission to RGCs and decreased miniature glutamatergic excitatory neurotransmission, reducing the excitability of the RGCs themselves, thus alleviating the excitability of RGCs in glaucomatous slices.
Conclusion: Quercetin may be a promising therapeutic agent for improving RGC survival and function in glaucomatous neurodegeneration. Quercetin exerted direct protective effects on RGCs by increasing inhibitory neurotransmission and decreasing excitatory neurotransmission to RGCs, thus reducing excitotoxic damage to those cells in glaucoma.
Keywords: excitatory synaptic inputs; glaucoma; inhibitory synaptic inputs; patch-clamp; quercetin; retinal ganglion cells.
Figures





Similar articles
-
Brimonidine enhances inhibitory postsynaptic activity of OFF- and ON-type retinal ganglion cells in a Wistar rat chronic glaucoma model.Exp Eye Res. 2019 Dec;189:107833. doi: 10.1016/j.exer.2019.107833. Epub 2019 Oct 13. Exp Eye Res. 2019. PMID: 31618613
-
Differential Modulation of the Excitatory and Inhibitory Synaptic Circuits of Retinal Ganglion Cells via Asiatic Acid in a Chronic Glaucoma Rat Model.J Clin Med. 2023 Jan 29;12(3):1056. doi: 10.3390/jcm12031056. J Clin Med. 2023. PMID: 36769706 Free PMC article.
-
Alpha7 nicotinic acetylcholine receptor agonist promotes retinal ganglion cell function via modulating GABAergic presynaptic activity in a chronic glaucomatous model.Sci Rep. 2017 May 11;7(1):1734. doi: 10.1038/s41598-017-02092-6. Sci Rep. 2017. PMID: 28496108 Free PMC article.
-
The photopic negative response (PhNR): measurement approaches and utility in glaucoma.Int Ophthalmol. 2020 Dec;40(12):3565-3576. doi: 10.1007/s10792-020-01515-0. Epub 2020 Jul 31. Int Ophthalmol. 2020. PMID: 32737731 Free PMC article. Review.
-
Electrophysiological assessment of retinal ganglion cell function.Exp Eye Res. 2015 Dec;141:164-70. doi: 10.1016/j.exer.2015.05.008. Epub 2015 May 18. Exp Eye Res. 2015. PMID: 25998495 Free PMC article. Review.
Cited by
-
Glymphatic imaging and modulation of the optic nerve.Neural Regen Res. 2022 May;17(5):937-947. doi: 10.4103/1673-5374.324829. Neural Regen Res. 2022. PMID: 34558505 Free PMC article.
-
Soluble tumor necrosis factor-alpha-induced hyperexcitability contributes to retinal ganglion cell apoptosis by enhancing Nav1.6 in experimental glaucoma.J Neuroinflammation. 2021 Aug 21;18(1):182. doi: 10.1186/s12974-021-02236-6. J Neuroinflammation. 2021. PMID: 34419081 Free PMC article.
-
Emerging strategies targeting genes and cells in glaucoma.Vision Res. 2025 Feb;227:108533. doi: 10.1016/j.visres.2024.108533. Epub 2024 Dec 6. Vision Res. 2025. PMID: 39644708 Review.
-
Effect of quercetin-loaded poly (lactic-co-glycolic) acid nanoparticles on lipopolysaccharide-induced memory decline, oxidative stress, amyloidogenesis, neurotransmission, and Nrf2/HO-1 expression.Heliyon. 2023 Dec 10;10(1):e23527. doi: 10.1016/j.heliyon.2023.e23527. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38169932 Free PMC article.
-
Beacon of Hope for Age-Related Retinopathy: Antioxidative Mechanisms and Pre-Clinical Trials of Quercetin Therapy.Antioxidants (Basel). 2025 May 8;14(5):561. doi: 10.3390/antiox14050561. Antioxidants (Basel). 2025. PMID: 40427443 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

