Characterization of Inflammation in Delayed Cortical Transplantation
- PMID: 31293384
- PMCID: PMC6603085
- DOI: 10.3389/fnmol.2019.00160
Characterization of Inflammation in Delayed Cortical Transplantation
Abstract
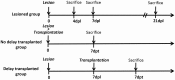
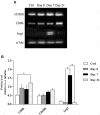
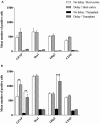
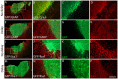
We previously reported that embryonic motor cortical neurons transplanted 1-week after lesion in the adult mouse motor cortex significantly enhances graft vascularization, survival, and proliferation of grafted cells, the density of projections developed by grafted neurons and improves functional repair and recovery. The purpose of the present study is to understand the extent to which post-traumatic inflammation following cortical lesion could influence the survival of grafted neurons and the development of their projections to target brain regions and conversely how transplanted cells can modulate host inflammation. For this, embryonic motor cortical tissue was grafted either immediately or with a 1-week delay into the lesioned motor cortex of adult mice. Immunohistochemistry (IHC) analysis was performed to determine the density and cell morphology of resident and peripheral infiltrating immune cells. Then, in situ hybridization (ISH) was performed to analyze the distribution and temporal mRNA expression pattern of pro-inflammatory or anti-inflammatory cytokines following cortical lesion. In parallel, we analyzed the protein expression of both M1- and M2-associated markers to study the M1/M2 balance switch. We have shown that 1-week after the lesion, the number of astrocytes, microglia, oligodendrocytes, and CD45+ cells were significantly increased along with characteristics of M2 microglia phenotype. Interestingly, the majority of microglia co-expressed transforming growth factor-β1 (TGF-β1), an anti-inflammatory cytokine, supporting the hypothesis that microglial activation is also neuroprotective. Our results suggest that the modulation of post-traumatic inflammation 1-week after cortical lesion might be implicated in the improvement of graft vascularization, survival, and density of projections developed by grafted neurons.
Keywords: cortical lesion; delay; embryonic transplantation; motor cortex; neuroinflammation.
Figures


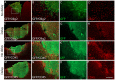
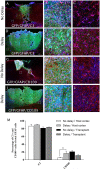

References
-
- Bernardino L., Xapelli S., Silva A. P., Jakobsen B., Poulsen F. R., Oliveira C. R., et al. (2005). Modulator effects of interleukin-1β and tumor necrosis factor-α on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J. Neurosci. 25, 6734–6744. 10.1523/JNEUROSCI.1510-05.2005 - DOI - PMC - PubMed
-
- Chao C. C., Hu S., Peterson P. K. (1995). Glia, cytokines, and neurotoxicity. Crit. Rev. Neurobiol. 9, 189–205. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

