Middle Ear Administration of a Particulate Chitosan Gel in an in vivo Model of Cisplatin Ototoxicity
- PMID: 31293387
- PMCID: PMC6603134
- DOI: 10.3389/fncel.2019.00268
Middle Ear Administration of a Particulate Chitosan Gel in an in vivo Model of Cisplatin Ototoxicity
Abstract
Background: Middle ear (intratympanic, IT) administration is a promising therapeutic method as it offers the possibility of achieving high inner ear drug concentrations with low systemic levels, thus minimizing the risk of systemic side effects and drug-drug interactions. Premature elimination through the Eustachian tube may be reduced by stabilizing drug solutions with a hydrogel, but this raises the secondary issue of conductive hearing loss.
Aim: This study aimed to investigate the properties of a chitosan-based particulate hydrogel formulation when used as a drug carrier for IT administration in an in vivo model of ototoxicity.
Materials and methods: Two particulate chitosan-based IT delivery systems, Thio-25 and Thio-40, were investigated in albino guinea pigs (n = 94). Both contained the hearing protecting drug candidate sodium thiosulfate with different concentrations of chitosan gel particles (25% vs. 40%). The safety of the two systems was explored in vivo. The most promising system was then tested in guinea pigs subjected to a single intravenous injection with the anticancer drug cisplatin (8 mg/kg b.w.), which has ototoxic side effects. Hearing status was evaluated with acoustically evoked frequency-specific auditory brainstem response (ABR) and hair cell counting. Finally, in vivo magnetic resonance imaging was used to study the distribution and elimination of the chitosan-based system from the middle ear cavity in comparison to a hyaluronan-based system.
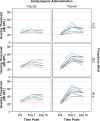
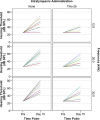
Results: Both chitosan-based IT delivery systems caused ABR threshold elevations (p < 0.05) that remained after 10 days (p < 0.05) without evidence of hair cell loss, although the elevation induced by Thio-25 was significantly lower than for Thio-40 (p < 0.05). Thio-25 significantly reduced cisplatin-induced ABR threshold elevations (p < 0.05) and outer hair cell loss (p < 0.05). IT injection of the chitosan- and hyaluronan-based systems filled up most of the middle ear space. There were no significant differences between the systems in terms of distribution and elimination.
Conclusion: Particulate chitosan is a promising drug carrier for IT administration. Future studies should assess whether the physical properties of this technique allow for a smaller injection volume that would reduce conductive hearing loss.
Keywords: auditory brainstem response; cisplatin; hair cell; hearing loss; intratympanic administration; magnetic resonance imaging; particulate chitosan; sodium thiosulfate.
Figures




References
-
- Arriaga M. A., Goldman S. (1998). Hearing results of intratympanic steroid treatment of endolymphatic hydrops. Laryngoscope 108 (11 Pt 1), 1682–1685. - PubMed
-
- Berglin C. E., Pierre P. V., Bramer T., Edsman K., Ehrsson H., Eksborg S., et al. (2011). Prevention of cisplatin-induced hearing loss by administration of a thiosulfate-containing gel to the middle ear in a guinea pig model. Cancer Chemother. Pharmacol. 68 1547–1556. 10.1007/s00280-011-1656-2 - DOI - PubMed
-
- Campbell K. C. M., Rybak L. P., Meech R. P., Hughes L. (1996). D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear. Res. 102 90–98. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

