Infant Trauma Alters Social Buffering of Threat Learning: Emerging Role of Prefrontal Cortex in Preadolescence
- PMID: 31293398
- PMCID: PMC6598593
- DOI: 10.3389/fnbeh.2019.00132
Infant Trauma Alters Social Buffering of Threat Learning: Emerging Role of Prefrontal Cortex in Preadolescence
Abstract
Within the infant-caregiver attachment system, the primary caregiver holds potent reward value to the infant, exhibited by infants' strong preference for approach responses and proximity-seeking towards the mother. A less well-understood feature of the attachment figure is the caregiver's ability to reduce fear via social buffering, commonly associated with the notion of a "safe haven" in the developmental literature. Evidence suggests this infant system overlaps with the neural network supporting social buffering (attenuation) of fear in the adults of many species, a network known to involve the prefrontal cortex (PFC). Here, using odor-shock conditioning in young developing rats, we assessed when the infant system transitions to the adult-like PFC-dependent social buffering of threat system. Rat pups were odor-shock conditioned (0.55 mA-0.6 mA) at either postnatal day (PN18; dependent on mother) or 28 (newly independent, weaned at PN23). Within each age group, the mother was present or absent during conditioning, with PFC assessment following acquisition using 14C 2-DG autoradiography and cue testing the following day. Since the human literature suggests poor attachment attenuates the mother's ability to socially buffer the infants, half of the pups at each age were reared with an abusive mother from PN8-12. The results showed that for typical control rearing, the mother attenuated fear in both PN18 and PN28 pups, although the PFC [infralimbic (IL) and ventral prelimbic (vPL) cortices] was only engaged at PN28. Abuse rearing completely disrupted social buffering of pups by the mother at PN18. The results from PN28 pups showed that while the mother modulated learning in both control and abuse-reared pups, the behavioral and PFC effects were attenuated after maltreatment. Our data suggest that pups transition to the adult-like PFC social support circuit after independence from the mother (PN28), and this circuit remains functional after early-life trauma, although its effectiveness appears reduced. This is in sharp contrast to the effects of early life trauma during infancy, where social buffering of the infant is more robustly impacted. We suggest that the infant social buffering circuit is disengaged by early-life trauma, while the adolescent PFC-dependent social buffering circuit may use a safety signal with unreliable safety value.
Keywords: early-life trauma; fear; infralimbic; prefrontal cortex; prelimbic; social buffering; social support; threat.
Figures
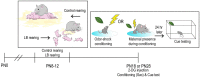
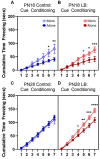
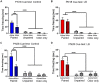
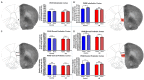

Similar articles
-
Neurobehavioral assessment of maternal odor in developing rat pups: implications for social buffering.Soc Neurosci. 2017 Feb;12(1):32-49. doi: 10.1080/17470919.2016.1159605. Epub 2016 Mar 22. Soc Neurosci. 2017. PMID: 26934130 Free PMC article.
-
Development of Threat Expression Following Infant Maltreatment: Infant and Adult Enhancement but Adolescent Attenuation.Front Behav Neurosci. 2019 Jun 25;13:130. doi: 10.3389/fnbeh.2019.00130. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31293397 Free PMC article.
-
The neurobiology of safety and threat learning in infancy.Neurobiol Learn Mem. 2017 Sep;143:49-58. doi: 10.1016/j.nlm.2016.10.015. Epub 2016 Nov 4. Neurobiol Learn Mem. 2017. PMID: 27826033 Free PMC article. Review.
-
Neurobiology of maternal regulation of infant fear: the role of mesolimbic dopamine and its disruption by maltreatment.Neuropsychopharmacology. 2019 Jun;44(7):1247-1257. doi: 10.1038/s41386-019-0340-9. Epub 2019 Feb 13. Neuropsychopharmacology. 2019. PMID: 30758321 Free PMC article.
-
Ecologically relevant neurobehavioral assessment of the development of threat learning.Learn Mem. 2016 Sep 15;23(10):556-66. doi: 10.1101/lm.042218.116. Print 2016 Oct. Learn Mem. 2016. PMID: 27634146 Free PMC article. Review.
Cited by
-
Neurobiology of Infant Fear and Anxiety: Impacts of Delayed Amygdala Development and Attachment Figure Quality.Biol Psychiatry. 2021 Apr 1;89(7):641-650. doi: 10.1016/j.biopsych.2020.08.020. Epub 2020 Aug 30. Biol Psychiatry. 2021. PMID: 33109337 Free PMC article. Review.
-
The lateral habenula integrates age and experience to promote social transitions in developing rats.Cell Rep. 2024 Aug 27;43(8):114556. doi: 10.1016/j.celrep.2024.114556. Epub 2024 Aug 1. Cell Rep. 2024. PMID: 39096491 Free PMC article.
-
Amygdala Allostasis and Early Life Adversity: Considering Excitotoxicity and Inescapability in the Sequelae of Stress.Front Hum Neurosci. 2021 Jun 1;15:624705. doi: 10.3389/fnhum.2021.624705. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34140882 Free PMC article.
-
Elevated infant cortisol is necessary but not sufficient for transmission of environmental risk to infant social development: Cross-species evidence of mother-infant physiological social transmission.Dev Psychopathol. 2020 Dec;32(5):1696-1714. doi: 10.1017/S0954579420001455. Dev Psychopathol. 2020. PMID: 33427190 Free PMC article.
-
Rodent models of early adversity: Impacts on developing social behavior circuitry and clinical implications.Front Behav Neurosci. 2022 Aug 4;16:918862. doi: 10.3389/fnbeh.2022.918862. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35990728 Free PMC article. Review.
References
-
- Almada R. C., Coimbra N. C., Brandão M. L. (2015). Medial prefrontal cortex serotonergic and GABAergic mechanisms modulate the expression of contextual fear: intratelencephalic pathways and differential involvement of cortical subregions. Neuroscience 284, 988–997. 10.1016/j.neuroscience.2014.11.001 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

