Gaboxadol Normalizes Behavioral Abnormalities in a Mouse Model of Fragile X Syndrome
- PMID: 31293404
- PMCID: PMC6603241
- DOI: 10.3389/fnbeh.2019.00141
Gaboxadol Normalizes Behavioral Abnormalities in a Mouse Model of Fragile X Syndrome
Abstract
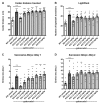
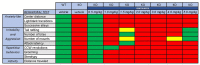
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability and autism. FXS is also accompanied by attention problems, hyperactivity, anxiety, aggression, poor sleep, repetitive behaviors, and self-injury. Recent work supports the role of γ-aminobutyric-acid (GABA), the primary inhibitory neurotransmitter in the brain, in mediating symptoms of FXS. Deficits in GABA machinery have been observed in a mouse model of FXS, including a loss of tonic inhibition in the amygdala, which is mediated by extrasynaptic GABAA receptors. Humans with FXS also show reduced GABAA receptor availability. Here, we sought to evaluate the potential of gaboxadol (also called OV101 and THIP), a selective and potent agonist for delta-subunit-containing extrasynaptic GABAA receptors (dSEGA), as a therapeutic agent for FXS by assessing its ability to normalize aberrant behaviors in a relatively uncharacterized mouse model of FXS (Fmr1 KO2 mice). Four behavioral domains (hyperactivity, anxiety, aggression, and repetitive behaviors) were probed using a battery of behavioral assays. The results showed that Fmr1 KO2 mice were hyperactive, had abnormal anxiety-like behavior, were more irritable and aggressive, and had an increased frequency of repetitive behaviors compared to wild-type (WT) littermates, which are all behavioral deficits reminiscent of individuals with FXS. Treatment with gaboxadol normalized all of the aberrant behaviors observed in Fmr1 KO2 mice back to WT levels, providing evidence of its potential benefit for treating FXS. We show that the potentiation of extrasynaptic GABA receptors alone, by gaboxadol, is sufficient to normalize numerous behavioral deficits in the FXS model using endpoints that are directly translatable to the clinical presentation of FXS. Taken together, these data support the future evaluation of gaboxadol in individuals with FXS, particularly with regard to symptoms of hyperactivity, anxiety, irritability, aggression, and repetitive behaviors.
Keywords: FMRP; GABA; OV101; THIP; aggression; anxiety; hyperactivity; stereotypy.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

