Exploring the Use of Pegylated Liposomal Doxorubicin (Caelyx®) as Pressurized Intraperitoneal Aerosol Chemotherapy
- PMID: 31293417
- PMCID: PMC6603215
- DOI: 10.3389/fphar.2019.00669
Exploring the Use of Pegylated Liposomal Doxorubicin (Caelyx®) as Pressurized Intraperitoneal Aerosol Chemotherapy
Abstract
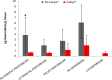
Background: Peritoneal carcinomatosis is a common metastatic pattern in ovarian, gastric, colorectal, and appendiceal cancer; systemic chemotherapy is the current standard of care for peritoneal metastatic disease; however, in a subset of patients its beneficial effect remains questionable. More effective perioperative chemotherapy is needed. Materials and methods: Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a new treatment that applies chemotherapeutic drugs into the peritoneal cavity as an aerosol under pressure. It's a safe and feasible approach that improves local bioavailability of chemotherapeutic drugs as compared with conventional intraperitoneal chemotherapy. Till now the drugs used in PIPAC for the treatment of the peritoneal carcinomatosis (PC) are cisplatin, doxorubicin, and oxaliplatin; as of yet, there are no in vivo data comparing different drug formulations and dosage schedules of PIPAC. Pegylated liposomal doxorubicin 1.5 mg/sm was aerosolized in PIPAC procedures. Results: Pharmacokinetics analysis of 10 procedures performed with conventional doxorubicin solution at the dose of 1.5 mg/m2 were compared to 15 procedures with the same dose of pegylated liposomal doxorubicin (PLD). Significant differences between experimental groups were detected by one-way ANOVA followed by Bonferroni correction; a p value < 0.05 was considered statistically significant. A statistically different doxorubicin tissue concentration was observed for the doxorubicin solution compared to pegylated liposomal doxorubicin in the right parietal peritoneum and right diaphragm. In the Caelyx® series a mean tissue concentration of 1.27 ± 1.33 mg/g was reported, while in the second one we registered a mean concentration of 3.1 ± 3.7 mg/g. Conclusions: The delivery of nano-particles in PIPAC was feasible, but pegylated liposomal concentrations are lower than standard doxorubicin formulation. Probably mechanical and physical properties of pressurized aerosol chemotherapy might alter their stability and cause structural disintegration.
Keywords: PIPAC; doxorubicin; locoregional chemotherapy; pegylated liposomes; peritoneal carcinomatosis.
Figures
Similar articles
-
Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis.World J Surg Oncol. 2016 Apr 29;14:128. doi: 10.1186/s12957-016-0892-7. World J Surg Oncol. 2016. PMID: 27125996 Free PMC article.
-
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Oxaliplatin, Cisplatin, and Doxorubicin in Patients with Peritoneal Carcinomatosis: An Open-Label, Single-Arm, Phase II Clinical Trial.Biomedicines. 2020 Apr 30;8(5):102. doi: 10.3390/biomedicines8050102. Biomedicines. 2020. PMID: 32365877 Free PMC article.
-
PIPAC-OV3: A multicenter, open-label, randomized, two-arm phase III trial of the effect on progression-free survival of cisplatin and doxorubicin as Pressurized Intra-Peritoneal Aerosol Chemotherapy (PIPAC) vs. chemotherapy alone in patients with platinum-resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer.Pleura Peritoneum. 2018 Sep 25;3(3):20180114. doi: 10.1515/pp-2018-0114. eCollection 2018 Sep 1. Pleura Peritoneum. 2018. PMID: 30911662 Free PMC article.
-
Pressurized intraperitoneal aerosol chemotherapy for recurrent ovarian, fallopian or primary peritoneal cancer with peritoneal carcinomatosis: a narrative review.Gland Surg. 2021 Mar;10(3):1244-1251. doi: 10.21037/gs-2019-ursoc-12. Gland Surg. 2021. PMID: 33842271 Free PMC article. Review.
-
Pressurized Intraperitoneal Aerosol Chemotherapy for Peritoneal Carcinomatosis in Colorectal Cancer Patients: A Systematic Review of the Evidence.Cancers (Basel). 2024 Oct 30;16(21):3661. doi: 10.3390/cancers16213661. Cancers (Basel). 2024. PMID: 39518099 Free PMC article. Review.
Cited by
-
Dendrimer Platforms for Targeted Doxorubicin Delivery-Physicochemical Properties in Context of Biological Responses.Int J Mol Sci. 2024 Jun 29;25(13):7201. doi: 10.3390/ijms25137201. Int J Mol Sci. 2024. PMID: 39000306 Free PMC article.
-
Doxorubicin or Epirubicin Versus Liposomal Doxorubicin Therapy-Differences in Cardiotoxicity.Cardiovasc Toxicol. 2025 Feb;25(2):248-268. doi: 10.1007/s12012-024-09952-4. Epub 2025 Jan 15. Cardiovasc Toxicol. 2025. PMID: 39810066 Review.
-
Increased Tissue Penetration of Doxorubicin in Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) after High-Intensity Ultrasound (HIUS).Int J Surg Oncol. 2019 Dec 12;2019:6185313. doi: 10.1155/2019/6185313. eCollection 2019. Int J Surg Oncol. 2019. PMID: 31915548 Free PMC article.
-
Pressurized intraperitoneal aerosol chemotherapy (PIPAC): updated systematic review using the IDEAL framework.Br J Surg. 2022 Dec 13;110(1):10-18. doi: 10.1093/bjs/znac284. Br J Surg. 2022. PMID: 36056893 Free PMC article. No abstract available.
-
Targeting Colorectal Cancer Cells with a Functionalised Calix[4]arene Receptor: Biophysical Studies.Molecules. 2022 Jan 14;27(2):510. doi: 10.3390/molecules27020510. Molecules. 2022. PMID: 35056825 Free PMC article.
References
-
- Alkhamesi N. A., Ridgway P. F., Ramwell A., McCullough P. W., Peck D. H., Darzi A. W. (2005). Peritoneal nebulizer: a novel technique for delivering intraperitoneal therapeutics in laparoscopic surgery to prevent locoregional recurrence. Surg. Endosc. 19, 1142–1146. 10.1007/s00464-004-2214-3 - DOI - PubMed
-
- Chattopadhyay S., Ehrman S. H., Bellare J., Venkataraman C. (2012). Morphology and bilayer integrity of small liposomes during aerosol generation by air-jet nebulisation | SpringerLink. J. Nanopart. Res. 14, 779. 10.1007/s11051-012-0779-7 - DOI