Efficacy and Safety of Brinzolamide as Add-On to Prostaglandin Analogues or β-Blocker for Glaucoma and Ocular Hypertension: A Systematic Review and Meta-Analysis
- PMID: 31293419
- PMCID: PMC6603202
- DOI: 10.3389/fphar.2019.00679
Efficacy and Safety of Brinzolamide as Add-On to Prostaglandin Analogues or β-Blocker for Glaucoma and Ocular Hypertension: A Systematic Review and Meta-Analysis
Abstract
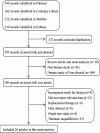
Background: Brinzolamide as a carbonic anhydrase inhibitor could be combined with other intraocular pressure (IOP) lowering drugs for glaucoma and ocular hypertension (OHT), but the efficacy was controversial. So, this study was used to assess the efficacy and safety of brinzolamide as add-on to prostaglandin analogues (PGAs) or β-blocker in treating patients with glaucoma or OHT who fail to adequately control IOP. Methods: We searched PubMed, Embase, MEDLINE, Cochrane Library, and clinicaltrials.gov from inception to October 4, 2018. Randomized controlled trials of brinzolamide as add-on to PGAs or β-blocker for glaucoma and OHT were included. Meta-analysis was conducted by RevMan 5.3 software. Results: A total of 26 trials including 5,583 patients were analyzed. Brinzolamide produced absolute reductions of IOP as an adjunctive therapy for patients with glaucoma or OHT. Brinzolamide and timolol were not significantly different in lowering IOP as add-on to PGAs (9 am: P = 0.07; 12 am: P = 0.66; 4 pm: P = 0.66). Likewise, brinzolamide was as effective as dorzolamide in depressing IOP (9 am: P = 0.59; 12 am: P = 0.94; 4 pm: P = 0.95). For the mean diurnal IOP at the end of treatment duration, there were no statistical differences in above comparisons (P > 0.05). Compared with brimonidine (b.i.d.), there was a significant reduction of IOP in brinzolamide (b.i.d.) at 9 am (P < 0.0001); however, the difference was cloudy in thrice daily subgroup (P = 0.44); at 12 am, brinzolamide (b.i.d.) was similar to brimonidine (b.i.d.) in IOP-lowering effect (P = 0.23), whereas brimonidine (t.i.d.) led to a greater effect than brinzolamide (t.i.d.) (P = 0.02). At 4 pm, brinzolamide (b.i.d.) was superior IOP-lowering effect compared with brimonidine (b.i.d.) (P = 0.0003); conversely, the effect in brinzolamide (t.i.d.) was lower than brimonidine (t.i.d.) (P < 0.0001). For the mean diurnal IOP, brinzolamide was lower in twice daily subgroup (P < 0.00001); brimonidine was lower in thrice daily subgroup (P < 0.00001). With regard to the safety, brinzolamide and dorzolamide had a higher incidence of taste abnormality; moreover, brinzolamide resulted in more frequent blurred vision; dorzolamide resulted in more frequent ocular discomfort and eye pain. Timolol resulted in more frequent blurred vision and less conjunctival hyperemia. Brimonidine resulted in more frequent ocular hyperemia. As to other adverse events (AEs) (conjunctivitis, eye pruritus, foreign body sensation in eyes, and treatment-related AEs), brinzolamide was similar to other three active comparators. Conclusions: Brinzolamide, as add-on to PGAs or β-blocker, significantly decreased IOP of patients with refractory glaucoma or OHT and the AEs were tolerable.
Keywords: brinzolamide; glaucoma; ocular hypertension; prostaglandin analogues; systematic review; β-blocker.
Figures




Similar articles
-
Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension.Ophthalmology. 2014 Dec;121(12):2348-55. doi: 10.1016/j.ophtha.2014.06.022. Epub 2014 Jul 23. Ophthalmology. 2014. PMID: 25064721 Clinical Trial.
-
A randomized, investigator-masked, 4-week study comparing timolol maleate 0.5%, brinzolamide 1%, and brimonidine tartrate 0.2% as adjunctive therapies to travoprost 0.004% in adults with primary open-angle glaucoma or ocular hypertension.Clin Ther. 2006 Apr;28(4):552-9. doi: 10.1016/j.clinthera.2006.04.007. Clin Ther. 2006. PMID: 16750466 Clinical Trial.
-
Brinzolamide : a review of its use in the management of primary open-angle glaucoma and ocular hypertension.Drugs Aging. 2003;20(12):919-47. doi: 10.2165/00002512-200320120-00008. Drugs Aging. 2003. PMID: 14565787 Review.
-
Brimonidine tartrate 0.15%, dorzolamide hydrochloride 2%, and brinzolamide 1% compared as adjunctive therapy to prostaglandin analogs.Ophthalmology. 2009 Sep;116(9):1719-24. doi: 10.1016/j.ophtha.2009.03.050. Epub 2009 Jul 9. Ophthalmology. 2009. PMID: 19592108 Clinical Trial.
-
Brimonidine and brinzolamide for treating glaucoma and ocular hypertension; a safety evaluation.Expert Opin Drug Saf. 2017 Sep;16(9):1071-1078. doi: 10.1080/14740338.2017.1346083. Epub 2017 Jul 6. Expert Opin Drug Saf. 2017. PMID: 28656780 Review.
Cited by
-
Sustained intraocular pressure-lowering effect and biocompatibility of a single subconjunctival administration of hydrogel-encapsulated nano-brinzolamide.J Mater Sci Mater Med. 2025 May 20;36(1):43. doi: 10.1007/s10856-025-06896-1. J Mater Sci Mater Med. 2025. PMID: 40392392 Free PMC article.
-
Topical carbonic anhydrase inhibitors and glaucoma in 2021: where do we stand?Br J Ophthalmol. 2022 Oct;106(10):1332-1337. doi: 10.1136/bjophthalmol-2021-319530. Epub 2021 Aug 25. Br J Ophthalmol. 2022. PMID: 34433550 Free PMC article. Review.
-
Co-delivery of brinzolamide and miRNA-124 by biodegradable nanoparticles as a strategy for glaucoma therapy.Drug Deliv. 2020 Dec;27(1):410-421. doi: 10.1080/10717544.2020.1731861. Drug Deliv. 2020. PMID: 32133894 Free PMC article.
References
-
- Aihara M., Adachi M., Matsuo H., Togano T., Fukuchi T., Sasaki N. (2017). Additive effects and safety of fixed combination therapy with 1% brinzolamide and 0.5% timolol versus 1% dorzolamide and 0.5% timolol in prostaglandin-treated glaucoma patients. Acta Ophthalmol. 95, e720–e726. 10.1111/aos.13401 - DOI - PMC - PubMed
-
- Alcon a.N.C. (2016). Comparison of Intraocular Pressure (IOP)-Lowering Efficacy and Safety of AZORGA® Ophthalmic Suspension and COSOPT® Ophthalmic Solution. (NCT02325518). Available online at: https://www.clinicaltrials.gov/
-
- Aung T., Laganovska G., Hernandez Paredes T. J., Branch J. D., Tsorbatzoglou A., Goldberg I. (2014). Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension. Ophthalmology 121, 2348–2355. 10.1016/j.ophtha.2014.06.022 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

