A Danshensu-Tetramethylpyrazine Conjugate DT-010 Overcomes Multidrug Resistance in Human Breast Cancer
- PMID: 31293428
- PMCID: PMC6606714
- DOI: 10.3389/fphar.2019.00722
A Danshensu-Tetramethylpyrazine Conjugate DT-010 Overcomes Multidrug Resistance in Human Breast Cancer
Abstract
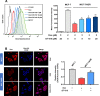
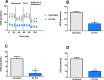
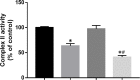
Background: We previously demonstrated that a Danshensu-Tetramethylpyrazine conjugate DT-010 enhanced anticancer effect of doxorubicin (Dox) in Dox-sensitive human breast cancer cells, and protected against Dox-induced cardiotoxicity. This work was designed to see whether DT-010 overcomes Dox resistance in resistant human breast cancer cells. Methods: The effects of DT-010, Dox or their combination on cell viability of Dox-resistant human breast cancer MCF-7/ADR cells were conducted using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Apoptosis was examined by flow cytometry after Annexin V-FITC/PI co-staining. Dox accumulation in MCF-7/ADR cells was detected by flow cytometry and fluorescence microscopy. A fluorometric multidrug resistance (MDR) assay kit was used to evaluate the effect of DT-010 on MDR transporter activity. P-glycoprotein (P-gp) expression and activity were analyzed by Western blot and rhodamine 123 (Rh123) efflux assay, respectively. The effects of DT-010 on glycolysis and mitochondrial stress were detected using an Extracellular Flux Analyzer. A Succinate Dehydrogenase Activity Assay kit was used to measure mitochondrial complex II activity. Results: At non-cytotoxic concentrations, DT-010 in combination with Dox led to a significant growth inhibition of MCF-7/ADR cells, suggesting a synergy between DT-010 and Dox to reverse Dox resistance. DT-010 restored Dox-mediated apoptosis and p53 induction in MCF-7/ADR cells. DT-010 increased Dox accumulation in MCF-7/ADR cells via inhibiting P-gp activity, but without changing P-gp expression. Further studies showed that DT-010 significantly inhibited glycolysis and mitochondrial function of MCF-7/ADR cells. Mitochondrial complex II activity was inhibited by DT-010 or DT-010/Dox combination, but not by Dox. The DT-010-mediated suppression of metabolic process may render cells more vulnerable to Dox treatment and thus result in enhanced efficacy. Conclusions: The results indicate that DT-010 overcomes Dox resistance in human breast cancer cells through a dual action via simultaneously inhibiting P-gp-mediated drug efflux and influencing metabolic process.
Keywords: P-glycoprotein; breast cancer; danshensu; glycolysis; resistance; tetramethylpyrazine.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

