Monitoring the Early Antiproliferative Effect of the Analgesic-Antitumor Peptide, BmK AGAP on Breast Cancer Using Intravoxel Incoherent Motion With a Reduced Distribution of Four b-Values
- PMID: 31293432
- PMCID: PMC6598093
- DOI: 10.3389/fphys.2019.00708
Monitoring the Early Antiproliferative Effect of the Analgesic-Antitumor Peptide, BmK AGAP on Breast Cancer Using Intravoxel Incoherent Motion With a Reduced Distribution of Four b-Values
Abstract
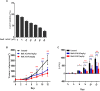
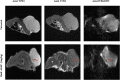
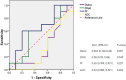
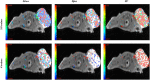
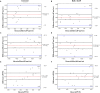
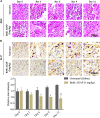
Background: The present study aimed to investigate the possibility of using intravoxel incoherent motion (IVIM) diffusion magnetic resonance imaging (MRI) to quantitatively assess the early therapeutic effect of the analgesic-antitumor peptide BmK AGAP on breast cancer and also evaluate the medical value of a reduced distribution of four b-values. Methods: IVIM diffusion MRI using 10 b-values and 4 b-values (0-1,000 s/mm2) was performed at five different time points on BALB/c mice bearing xenograft breast tumors treated with BmK AGAP. Variability in Dslow, Dfast, PF, and ADC derived from the set of 10 b-values and 4 b-values was assessed to evaluate the antitumor effect of BmK AGAP on breast tumor. Results: The data showed that PF values significantly decreased in rBmK AGAP-treated mice on day 12 (P = 0.044). PF displayed the greatest AUC but with a poor medical value (AUC = 0.65). The data showed no significant difference between IVIM measurements acquired from the two sets of b-values at different time points except in the PF on the day 3. The within-subject coefficients of variation were relatively higher in Dfast and PF. However, except for a case noticed on day 0 in PF measurements, the results indicated no statistically significant difference at various time points in the rBmK AGAP-treated or the untreated group (P < 0.05). Conclusion: IVIM showed poor medical value in the early evaluation of the antiproliferative effect of rBmK AGAP in breast cancer, suggesting sensitivity in PF. A reduced distribution of four b-values may provide remarkable measurements but with a potential loss of accuracy in the perfusion-related parameter PF.
Keywords: Buthus martensii Karsch; analgesic–antitumor peptide; breast neoplasms; intravoxel incoherent motion; magnetic resonance imaging.
Figures

References
-
- Che S., Zhao X., Ou Y., Li J., Wang M., Wu B., et al. (2016). Role of the intravoxel incoherent motion diffusion weighted imaging in the pre-treatment prediction and early response monitoring to neoadjuvant chemotherapy in locally advanced breast cancer. Medicine 95:e2420. 10.1097/MD.0000000000002420 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical

