Metabolic Cost of Activation and Mechanical Efficiency of Mouse Soleus Muscle Fiber Bundles During Repetitive Concentric and Eccentric Contractions
- PMID: 31293438
- PMCID: PMC6599155
- DOI: 10.3389/fphys.2019.00760
Metabolic Cost of Activation and Mechanical Efficiency of Mouse Soleus Muscle Fiber Bundles During Repetitive Concentric and Eccentric Contractions
Abstract
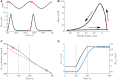
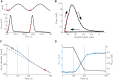
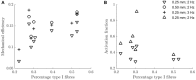
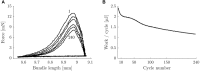
Currently available data on the energetics of isolated muscle preparations are based on bouts of less than 10 muscle contractions, whereas metabolic energy consumption is mostly relevant during steady state tasks such as locomotion. In this study we quantified the energetics of small fiber bundles of mouse soleus muscle during prolonged (2 min) series of contractions. Bundles (N = 9) were subjected to sinusoidal length changes, while measuring force and oxygen consumption. Stimulation (five pulses at 100 Hz) occurred either during shortening or during lengthening. Movement frequency (2-3 Hz) and amplitude (0.25-0.50 mm; corresponding to ± 4-8% muscle fiber strain) were close to that reported for mouse soleus muscle during locomotion. The experiments were performed at 32°C. The contributions of cross-bridge cycling and muscle activation to total metabolic energy expenditure were separated using blebbistatin. The mechanical work per contraction cycle decreased sharply during the first 10 cycles, emphasizing the importance of prolonged series of contractions. The mean ± SD fraction of metabolic energy required for activation was 0.37 ± 0.07 and 0.56 ± 0.17 for concentric and eccentric contractions, respectively (both 0.25 mm, 2 Hz). The mechanical efficiency during concentric contractions increased with contraction velocity from 0.12 ± 0.03 (0.25 mm 2 Hz) to 0.15 ± 0.03 (0.25 mm, 3 Hz) and 0.16 ± 0.02 (0.50 mm, 2 Hz) and was -0.22 ± 0.08 during eccentric contractions (0.25 mm, 2 Hz). The percentage of type I fibers correlated positively with mechanical efficiency during concentric contractions, but did not correlate with the fraction of metabolic energy required for activation.
Keywords: blebbistatin; cross-bridge cycling; mechanical efficiency; mouse soleus muscle; muscle activation; oxygen consumption.
Figures




References
LinkOut - more resources
Full Text Sources

