Goethite Hinders Azo Dye Bioreduction by Blocking Terminal Reductive Sites on the Outer Membrane of Shewanella decolorationis S12
- PMID: 31293561
- PMCID: PMC6604703
- DOI: 10.3389/fmicb.2019.01452
Goethite Hinders Azo Dye Bioreduction by Blocking Terminal Reductive Sites on the Outer Membrane of Shewanella decolorationis S12
Abstract
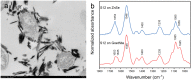
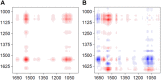
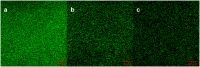
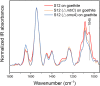
Iron (hydr)oxides are the most ubiquitous Fe(III)-containing minerals in the near-surface environments and can regulate organic pollutant biotransformation by participating in bacterial extracellular electron transfer under anaerobic conditions. Mechanisms described so far are based on their redox properties in bacterial extracellular respiration. Here, we find that goethite, a typical iron (hydr)oxide, inhibits the bioreduction of different polar azo dyes by Shewanella decolorationis S12 not through electron competition, but by the contact of its surface Fe(III) with the bacterial outer surface. Through the combined results of attenuated total reflectance (ATR) Fourier transform infrared spectroscopy, two-dimensional correlation spectroscopy, and confocal laser scanning microscope, we found that the outer membrane proteins MtrC and OmcA of strain S12 are key binding sites for goethite surface. Meanwhile, they were identified as the important reductive terminals for azo dyes. These results suggest that goethite may block the terminal reductive sites of azo dyes on the bacterial outer membrane to inhibit their bioreduction. This discovered role of goethite in bioreduction provides new insight into the microbial transformation processes of organic pollutants in iron (hydr)oxide-containing environments.
Keywords: Shewanella decolorationis S12; azo dye; bioreduction; goethite; mineral interface.
Figures


References
-
- Amonette J. E., Workman D. J., Kennedy D. W., Fruchter J. S., Gorby Y. A. (2000). Dechlorination of carbon tetrachloride by Fe(II) associated with goethite. Environ. Sci. Technol. 34 4606–4613. 10.1021/es9913582 - DOI
-
- Atkinson R. J., Posner A. M., Quirk J. P. (1967). Adsorption of potential-determining ions at the ferric oxide-aqueous electrolyte interface. J. Phys. Chem. 71 550–558. 10.1021/j100862a014 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

