Alpha-1-Antitrypsin Enhances Primary Human Macrophage Immunity Against Non-tuberculous Mycobacteria
- PMID: 31293581
- PMCID: PMC6606736
- DOI: 10.3389/fimmu.2019.01417
Alpha-1-Antitrypsin Enhances Primary Human Macrophage Immunity Against Non-tuberculous Mycobacteria
Abstract
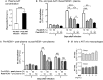
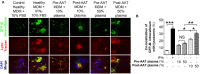
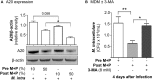
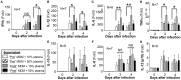
Rationale: The association between non-tuberculous mycobacterial lung disease and alpha-1-antitrypsin (AAT) deficiency is likely due, in part, to underlying emphysema or bronchiectasis. But there is increasing evidence that AAT itself enhances host immunity against microbial pathogens and thus deficiency could compromise host protection. Objectives: The goal of this project is to determine if AAT could augment macrophage activity against non-tuberculous mycobacteria. Methods: We compared the ability of monocyte-derived macrophages cultured in autologous plasma that were obtained immediately before and soon after AAT infusion-given to individuals with AAT deficiency-to control an ex vivo Mycobacterium intracellulare infection. Measurements and Main Results: We found that compared to pre-AAT infused monocyte-derived macrophages plus plasma, macrophages, and contemporaneous plasma obtained after a session of AAT infusion were significantly better able to control M. intracellulare infection; the reduced bacterial burden was linked with greater phagosome-lysosome fusion and increased autophagosome formation/maturation, the latter due to AAT inhibition of both M. intracellulare-induced nuclear factor-kappa B activation and A20 expression. While there was a modest increase in apoptosis in the M. intracellulare-infected post-AAT infused macrophages and plasma, inhibiting caspase-3 in THP-1 cells, monocyte-derived macrophages, and alveolar macrophages unexpectedly reduced the M. intracellulare burden, indicating that apoptosis impairs macrophage control of M. intracellulare and that the host protective effects of AAT occurred despite inducing apoptosis. Conclusion: AAT augments macrophage control of M. intracellulare infection through enhancing phagosome-lysosome fusion and autophagy.
Keywords: autophagy; mycobacteria; nuclear factor-kappa B; phagosome-lysosome fusion; serine protease inhibitor.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

