Pemphigus: Current and Future Therapeutic Strategies
- PMID: 31293582
- PMCID: PMC6603181
- DOI: 10.3389/fimmu.2019.01418
Pemphigus: Current and Future Therapeutic Strategies
Abstract
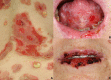
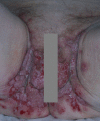
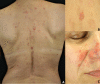
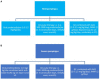
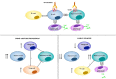
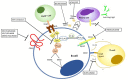
Pemphigus encompasses a heterogeneous group of autoimmune blistering diseases, which affect both mucous membranes and the skin. The disease usually runs a chronic-relapsing course, with a potentially devastating impact on the patients' quality of life. Pemphigus pathogenesis is related to IgG autoantibodies targeting various adhesion molecules in the epidermis, including desmoglein (Dsg) 1 and 3, major components of desmosomes. The pathogenic relevance of such autoantibodies has been largely demonstrated experimentally. IgG autoantibody binding to Dsg results in loss of epidermal keratinocyte adhesion, a phenomenon referred to as acantholysis. This in turn causes intra-epidermal blistering and the clinical appearance of flaccid blisters and erosions at involved sites. Since the advent of glucocorticoids, the overall prognosis of pemphigus has largely improved. However, mortality persists elevated, since long-term use of high dose corticosteroids and adjuvant steroid-sparing immunosuppressants portend a high risk of serious adverse events, especially infections. Recently, rituximab, a chimeric anti CD20 monoclonal antibody which induces B-cell depletion, has been shown to improve patients' survival, as early rituximab use results in higher disease remission rates, long term clinical response and faster prednisone tapering compared to conventional immunosuppressive therapies, leading to its approval as a first line therapy in pemphigus. Other anti B-cell therapies targeting B-cell receptor or downstream molecules are currently tried in clinical studies. More intriguingly, a preliminary study in a preclinical mouse model of pemphigus has shown promise regarding future therapeutic application of Chimeric Autoantibody Receptor T-cells engineered using Dsg domains to selectively target autoreactive B-cells. Conversely, previous studies from our group have demonstrated that B-cell depletion in pemphigus resulted in secondary impairment of T-cell function; this may account for the observed long-term remission following B-cell recovery in rituximab treated patients. Likewise, our data support the critical role of Dsg-specific T-cell clones in orchestrating the inflammatory response and B-cell activation in pemphigus. Monitoring autoreactive T-cells in patients may indeed provide further information on the role of these cells, and would be the starting point for designating therapies aimed at restoring the lost immune tolerance against Dsg. The present review focuses on current advances, unmet challenges and future perspectives of pemphigus management.
Keywords: BTK inhibitors; CAAR T-cell; anti-CD 20 antibodies; neonatal Fc receptor (FcRn); pemphigus; rituximab.
Figures

References
-
- Vu TN, Lee TX, Ndoye A, Shultz LD, Pittelkow MR, Dahl MV, et al. The pathophysiological significance of nondesmoglein targets of pemphigus autoimmunity. Development of antibodies against keratinocyte cholinergic receptors in patients with pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol. (1998) 134:971–80. 10.1001/archderm.134.8.971 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

